Post by: theanonymous on January 17, 2013, 12:07:56 AM
The structure (below) I drew at the bottom is wrong :(
Here's the SMILES string...
CC1=CCC=CC1
In the first electron transfer, the carbon to which the sodium electron adds creates a carbanion and the carbon "para" to the carbanion is a carbon radical which becomes a carbanion upon electron transfer from the second sodium atom. Since the carbanion is negatively charged, it would not be low in energy if it formed on a carbon bonded to an alkyl group (an electron pump). This results in the alkyl group being bonded to a double bond carbon (sp2 carbon) and not to an sp3 carbon.
But what's the answer?
Post by: Calicum on January 17, 2013, 12:20:52 AM
Post by: theanonymous on January 17, 2013, 12:33:22 AM
Birch Reduction
Can you show me using this link? http://cactus.nci.nih.gov/translate/
Post by: discodermolide on January 17, 2013, 12:44:20 AM
Post by: theanonymous on January 17, 2013, 12:51:17 AM
Look here: http://en.wikipedia.org/wiki/Birch_reduction (http://en.wikipedia.org/wiki/Birch_reduction)
I looked at that like 4 min ago.
That's what I did and I still got it wrong...
Post by: discodermolide on January 17, 2013, 12:56:44 AM
Post by: Dan on January 17, 2013, 03:28:21 AM
That's what I did and I still got it wrong...
You replaced the isopropyl group with a methyl group.
Post by: Borek on January 17, 2013, 03:57:49 AM
Here's the SMILES string...
CC1=CCC=CC1
If you know SMILES, why don't you use [smiles]CC1=CCC=CC1[/smiles] tags?
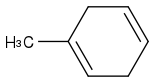
Post by: curiouscat on January 17, 2013, 06:31:24 AM
Here's the SMILES string...
CC1=CCC=CC1
If you know SMILES, why don't you use [smiles]CC1=CCC=CC1[/smiles] tags?
Adding frills
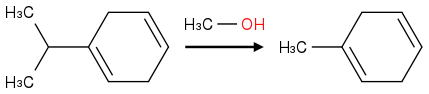
Post by: theanonymous on January 17, 2013, 08:01:18 AM
How about this product?
Oh :o
wait...
Post by: theanonymous on January 17, 2013, 08:06:43 AM
That's what I did and I still got it wrong...
You replaced the isopropyl group with a methyl group.
Ohhhh... for some reason I thought the 2 extra lines on the isopropyl group were implied Hydrogens...
Thanks a bunch!!
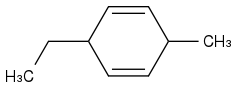
Post by: theanonymous on January 17, 2013, 08:07:03 AM
How about this product?
That makes more sense!
Thanks!!
Post by: theanonymous on January 17, 2013, 08:09:16 AM
Do I do the same thing with the Birch Reduction?
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi11.photobucket.com%2Falbums%2Fa179%2Fslourg%2FChemistry2_zpsb4a53810.png&hash=49a34356161aee9986f47b1c7a4591214b0cb8ed)
Like this?
Because this is what I had in my notes...
In the first electron transfer, the carbon to which the sodium electron adds creates a carbanion and the carbon "para" to the carbanion is a carbon radical which becomes a carbanion upon electron transfer from the second sodium atom. Since the carbanion is negatively charged, it would not be low in energy if it formed on a carbon bonded to an alkyl group (an electron pump). This results in the alkyl group being bonded to a double bond carbon (sp2 carbon) and not to an sp3 carbon.
Post by: theanonymous on January 17, 2013, 08:21:18 AM
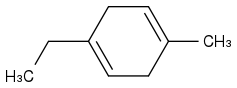
Post by: theanonymous on January 17, 2013, 08:27:56 AM
I'm not sure about this one though...
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi11.photobucket.com%2Falbums%2Fa179%2Fslourg%2FChemistry3NEW_zps04651a84.png&hash=07e4fd94936ddedfd4c8ca453812a211f8102978)
I got:
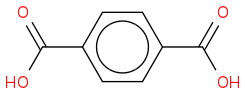
Does that look right?
Because if the alkyl group possesses a benzylic hydrogen then that carbon will be oxidized to a carboxylic acid.
So if THIS one is right, then what's the product for this one? o.O
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi11.photobucket.com%2Falbums%2Fa179%2Fslourg%2FChemistry4_zps68de6e04.png&hash=ee0f0569ba117d7977d129907a405ccb91e4cad3)
I tried doing
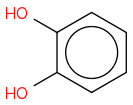 but Webassign said I was wrong...
but Webassign said I was wrong...
Post by: sjb on January 17, 2013, 10:25:38 AM
I think I got the one above right...
I'm not sure about this one though...
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi11.photobucket.com%2Falbums%2Fa179%2Fslourg%2FChemistry3NEW_zps04651a84.png&hash=07e4fd94936ddedfd4c8ca453812a211f8102978)
I got:
Does that look right?
Because if the alkyl group possesses a benzylic hydrogen then that carbon will be oxidized to a carboxylic acid.
So if THIS one is right, then what's the product for this one? o.O
I tried doingbut Webassign said I was wrong...
Where is the carboxylic acid functionality in your product?