Post by: confusedstud on February 24, 2013, 10:59:39 PM
Post by: argulor on February 24, 2013, 11:19:46 PM
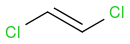 and
and 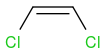 .
.
Post by: confusedstud on February 25, 2013, 01:48:39 AM
Post by: argulor on February 25, 2013, 10:50:37 AM
Hydrogen bonding is the main dominant force, but what is the link between this and net dipole moment? Also, due to them being able to rotate shouldn't the net dipole moment always fluctuate?
Yes, and essentially we measure the average dipole moment of this rotation.
Post by: confusedstud on February 25, 2013, 11:07:15 AM
Hydrogen bond donors and acceptors will almost always be active (at STP) regardless of symmetry.Hydrogen bonding is the main dominant force, but what is the link between this and net dipole moment? Also, due to them being able to rotate shouldn't the net dipole moment always fluctuate?
Yes, and essentially we measure the average dipole moment of this rotation.
Oh I understand this better now. But what do you mean by "hydrogen bond donors and acceptors will almost always be active"? Also, since they are constantly fluctuating how is that we can measure them to be considered polar? Thanks for the help :)
Post by: opsomath on February 25, 2013, 03:53:12 PM
Post by: Babcock_Hall on February 25, 2013, 03:53:34 PM
Post by: confusedstud on February 25, 2013, 10:57:54 PM
In any molecule with hydrogen bonding ability, forces due to the overall dipole moment can be almost completely ignored. What matters much more is local dipoles, ie, polarized bonds.
does this include diketones too? Because if I had a polar solvent, say HCl and I were to dissolve my diol in this. So how do we explain that the diol is polar too so it can dissolve in a polar solvent like HCl?
Post by: Babcock_Hall on February 26, 2013, 09:37:40 AM
Post by: confusedstud on February 26, 2013, 11:22:24 AM
Thanks for the help :)
Post by: Needaask on March 07, 2013, 04:01:47 AM
@OP, How are the intermolecular forces in a diketone like or unlike a diol?
hi i was going through the forum posts and this is also one of the chapters that i don't quite understand.
but in the solution since the maximum number of H bonds doesn't change so does it mean that diketone is infinitely soluble in HCl? I'm thinking since the number of H bonds doesn't change so the intermolecular interactions are the same so they should be infinitely soluble in each other?