Post by: Messi on March 12, 2013, 10:26:40 PM
I have a few reactions I am thinking of, and am wondering if they woudl theoretically work... They are fairly basic reactions... so I think they would, but a simply "yes" or "no" would be greatly appreciated! For reaction #1 shown below would it work? For reaction #2, how can I form the corresponding amine?
The picture is shown below (took a picture of my drawing)
Thanks!
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi50.tinypic.com%2F153mqts.jpg&hash=ba083957f32c61c3f7dab123138f2dea0b3c90e6)
Post by: orgopete on March 13, 2013, 01:21:25 AM
2) What is the "?"?
Post by: camptzak on March 13, 2013, 01:43:08 AM
Post by: discodermolide on March 13, 2013, 01:55:18 AM
Post by: Dan on March 13, 2013, 03:56:52 AM
Reaction of TsCl with an alcohol ROH gives a sulfonate (ArSO3R), not a sulfone (ArSO2R):
ROH + TsCl :rarrow: ROTs + HCl
Post by: Messi on March 13, 2013, 07:45:22 AM
Dan, thanks for this, I simply forgot to include the "O" when I drew my ROTs.
Orgopete, thanks for your reply as well. Yes, the dialkylated amine would be seen as a major product. Any ideas how I could simply get the mono-substituted amine (what reaction)? Also, I guess when I do my reaction, I'll have to a run a column to isolate my mono-substituted product.
The question mark in the second reaction was me not being sure what reagents to use to form my desired product. Could you guys give me a hand in that regard?
Thanks!
On a side note, does the reaction below work? I am following orgopete's advice and looking at other ways of creating my amine.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi48.tinypic.com%2F2zghz49.jpg&hash=7e29f40c4eae2dd7d568028e36f3b42bc4d3681f)
Post by: discodermolide on March 13, 2013, 09:51:23 AM
Post by: Messi on March 13, 2013, 08:18:04 PM
When you say reductive amination, I understand that, but I don't think I have any chemicals that will allow me to make my wanted amine with that reaction mechanism.
1) Would work, a little sloppy, but I'm sure the product could be found among the dialkylated amine.
What eluent system should I use to isolate my monoalkylated amine?
2) What is the "?"?
I don't know what the "?" should be. Could you give me a hand? :)
Post by: Dan on March 14, 2013, 04:19:33 AM
I am confused. I just want to make my amine so I can continue my reactions and form something else after that!
The main point that you need to understand is that alkylation of amines by SN2 reactions with alkyl halides or (alkyl sulfonates) is not a good general method because it can be difficult to control the product distribution - you often get alkylation. Think about mixing MeI and ammonia - you will get a mixture of methylamine, dimethylamine, trimethylamine and tetramethylammonium iodide.
This is a classic problem discuessed in any organic textbooks, and there are several well established workarounds including: Azide displacement, Delepine reaction, Gabriel synthesis, reductive amination, reduction of an amide, Curtius rearrangement and Hoffman rearrangement. Look these up for some inspiration for your problem.
Quote
When you say reductive amination, I understand that, but I don't think I have any chemicals that will allow me to make my wanted amine with that reaction mechanism.
Design the route, then buy in what you need. The materials required for the purification of a messy SN2 reaction may cost more than just buying the reagents for a well planned synthesis.
Quote
What eluent system should I use to isolate my monoalkylated amine?
That can only be determined experimentally.
Post by: Messi on March 14, 2013, 11:09:08 AM
On a side note, anyone know what reagents I need to use below to form my amine with a halide group on it?
*Look @ picture below* What could I use as the question mark?
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi50.tinypic.com%2Fa0vtxc.png&hash=a7fd658e3d82f5b9385278450a028e69b8bd0e59)
Post by: Dan on March 14, 2013, 12:09:10 PM
Post by: Messi on March 14, 2013, 12:36:13 PM
I wrote my reaction mechanism below. I am not sure how I can reduce the carbonyl without affecting the chloride?
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi47.tinypic.com%2F23mkhmc.jpg&hash=c329765ad44220d1572c8747179c418212a2e7d8)
Post by: discodermolide on March 14, 2013, 12:40:26 PM
Secondly there are ways of reducing an acid/ester to the corresponding alcohol. From there you can obtain the halide.
Post by: Messi on March 14, 2013, 12:51:13 PM
How does my refined reaction mechanism below look?
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi50.tinypic.com%2Fjhqvxi.jpg&hash=010437b52be7cff2e2bdd0c30f2ee6ae2cdfac15)
Post by: Dan on March 14, 2013, 01:01:39 PM
Post by: Messi on March 14, 2013, 01:11:01 PM
Finally got it! Picture below!
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi48.tinypic.com%2Fk3a97o.jpg&hash=ba8b5a3b7879f8a50c3cc67e80a9b4a6d3c64701)
Post by: discodermolide on March 14, 2013, 01:17:06 PM
You need to protect the nitrogen first. Using Schotten-Baumann conditions.
Post by: Messi on March 14, 2013, 01:36:48 PM
Btw guys, I really appreciate the *delete me*
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi45.tinypic.com%2Fb7gc5d.jpg&hash=0ff0af74befb55a51e8fa07a3c9222dc567a8757)
Post by: discodermolide on March 14, 2013, 01:45:10 PM
Make the ester and then try LiBH4 reduction, the Boc should survive.
You could also make the acid chloride, do a Rosenmund reduction and reduce the aldehyde to the alcohol.
Post by: Messi on March 14, 2013, 01:49:03 PM
Post by: discodermolide on March 14, 2013, 01:51:50 PM
I have references but unfortunately no experimental that I have found yet on this PC.
Post by: Dan on March 14, 2013, 02:05:47 PM
1. The Boc group replaces the H in the NH, i.e. R2NH :rarrow: R2NBoc
2. Boc is not usually written backwards, i.e. R2NBoc and BocNR2 look normal, but coBNR2 is never used. This is true of all abbreviated groups that do not show atom connecivity.
3. HCl will not displace a tosylate. You need a nucleophile, i.e. chloride ion. Bromide or iodide would be better (more nucleophilic). HCl will just remove the Boc group.
I see what you're trying to do, and it seems feasable. However, it can be done more efficiently:
You can "protect" the amine by forming the hydrobromide salt:
![[Br-].OCC1CCC[NH2+]1](https://www.chemicalforums.com/SMILES/4c0ebdc28d6df783ab5a.png)
Then heat it with PBr3 to get the alkyl bromide.
Alternatively, you could make the hydrochloride salt and then make the alkyl chloride.
These compounds would normally be handled as salts or N-protected because they are relatively unstable in free-base form.
Post by: Messi on March 14, 2013, 02:11:00 PM
Make the ester and then try LiBH4 reduction, the Boc should survive.
This? Shown below? I used LiBH4
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi48.tinypic.com%2F2hguwyd.jpg&hash=da2cf8f1047c7ca570659a169db3fea0bbe31a94)
You could also make the acid chloride, do a Rosenmund reduction and reduce the aldehyde to the alcohol.
That's an extra step because I need to turn my proline into an acyl chloride then into a primary alcohol when I could simply turn my proline carboxylic acid functional group directly into a primary alcohol via reduction :)
Post by: Messi on March 14, 2013, 02:14:22 PM
A couple of things:
1. The Boc group replaces the H in the NH, i.e. R2NH :rarrow: R2NBoc
2. Boc is not usually written backwards, i.e. R2NBoc and BocNR2 look normal, but coBNR2 is never used. This is true of all abbreviated groups that do not show atom connecivity.
3. HCl will not displace a tosylate. You need a nucleophile, i.e. chloride ion. Bromide or iodide would be better (more nucleophilic). HCl will just remove the Boc group.
Thanks Dan! I appreciate this!
I see what you're trying to do, and it seems feasable. However, it can be done more efficiently:
You can "protect" the amine by forming the hydrobromide salt:
Then heat it with PBr3 to get the alkyl bromide.
Alternatively, you could make the hydrochloride salt and then make the alkyl chloride.
These compounds would normally be handled as salts or N-protected because they are relatively unstable in free-base form.
I like this one, nice quick and easy! I'll try it that way and the other way and see what I get it! I am mainly trying to get lab experience at this stage of my career. Thanks for the *delete me*
Post by: discodermolide on March 14, 2013, 02:18:21 PM
I have a procedure for pyroglutamate (ethyl ester) to the alcohol with LiBH4.
Post by: Messi on March 15, 2013, 07:34:02 PM
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0530 (http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0530)
For the set-up, it says: An oven-dried three-necked flask equipped with a mechanical stirrer, a Friedrich condenser, and a nitrogen-inlet tube. Based on this, I am not sure how I will be adding my proline to my mixture. Proline is a solid, and I was told to use a plastic sleeve to add my proline. How exactly am I going to do that! Would anyone mind telling me how I will set-up for such a reaction?
Would the set-up look like something shown below?
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi45.tinypic.com%2F1491d86.png&hash=481b587e05a4acb12ec3dc7ef6bbc305b6dd3096)
Post by: Corribus on March 15, 2013, 09:29:27 PM
Based on this, I am not sure how I will be adding my proline to my mixture. Proline is a solid, and I was told to use a plastic sleeve to add my proline. How exactly am I going to do that!If the whole reaction vessel is under positive pressure, you can remove the stopper and add it quickly, followed by replacing the stopper. You will want to have nitrogen constantly cycling (using a septum and needle, say) afterwards to remove residual oxygen. Just be careful you don't blow off all your solvent. But the better way to do it is to do a cannula transfer of dissolved proline, supposing it is soluble in the reaction solvent. Even if it isn't, you can usually transfer a suspension over this way if the reactant is in excess.
This will all be much easier to do on a Schlenk line.
Post by: Messi on March 15, 2013, 09:49:20 PM
I do have access to a Schlenk line, and I do agree that doing a cannula transfer would be much easier (just saw a video on the process - looks very neat). Regarding having nitrogen or argon constantly cycling via a septum and needle, where should I place my septum and needle in my previously posted picture?
Also, as a side note, how should I measure my desired amount of LiAlH4. I can't simply use a balance and expose my LiAlH4 to air... wouldn't that be dangerous!
Post by: discodermolide on March 15, 2013, 10:04:09 PM
Where is the mechanical stirrer?
Is your LiAlH4 in solution or a solid?
What scale are you doing this reaction on?
Post by: Messi on March 15, 2013, 10:31:25 PM
That is not a three-necked flask.
I thought my apparatus would have the same effect as a three-necked flask.
Where is the mechanical stirrer?
I was going to use a magnetic stir bar.
Is your LiAlH4 in solution or a solid?
A solid. So, I will add my THF in my RBF, then add my LiAlH4 after. Not sure how I will weigh my solid however.
What scale are you doing this reaction on?
Small scale. Probably on 50-60 mmol of LiAlH4
Anything wrong with my set-up Discodermolide? I have big plans once I create my product(s) :)
Post by: discodermolide on March 15, 2013, 10:51:07 PM
Personally I would use a mechanical stirrer, it is more efficient, especially for the work-up.
How much THF are you going to use?
Post by: Messi on March 15, 2013, 10:57:44 PM
You weigh your LiAlH4 in the RBF with the other attachments already on the flask. You can do this under nitrogen or argon. Then you add the THF. Then you can add the proline with a spatula still maintaining a positive nitrogen pressure.
Just realized this, thanks!
How much THF are you going to use?
Probably ~60mL
If I do use a three-necked, round-bottomed flask fitted with a reflux condenser (CaCl2 drying tube), addition funnel, and a mechanical stirrer, would I simply add a septa and needle feeding in nitrogen from the schlenk line at the top of the reflux condenser?
Post by: discodermolide on March 15, 2013, 11:05:19 PM
Or you can use septa and needles if you wish. I personally don't like them especially for reflux. But it's up to you.
You will have around a 1.0molar solution of hydride, would it not be better to use the solution from Aldrich which I seem to remember is about 1.0molar in THF?
Can you be sure of your quality of LiAlH4? Try and get an unopened tin.
Post by: Messi on March 15, 2013, 11:15:08 PM
Can you be sure of your quality of LiAlH4? Try and get an unopened tin.
Yup, I had checked earlier today, I have an unopened tin!
In the procedure I was following on http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0530 (http://www.orgsyn.org/orgsyn/prep.asp?prep=cv7p0530), how come there is no mention of adding ethyl acetate to destroy excess LiAlH4 when I am about to isolate/purify my product?
Post by: discodermolide on March 15, 2013, 11:26:25 PM
Post by: Messi on March 16, 2013, 01:39:51 PM
Post by: discodermolide on March 16, 2013, 01:45:42 PM
You do have some supervision for this reaction? Read up about the properties of LiAlH4 especially the hazards.
Post by: Messi on March 16, 2013, 02:52:20 PM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi45.tinypic.com%2F2qs9aa1.jpg&hash=07b4b81b2e59ca9ef69b08f796eef45118326aeb)
Post by: orgopete on March 16, 2013, 02:59:49 PM
Post by: discodermolide on March 16, 2013, 03:00:26 PM
Good luck.
Post by: Messi on March 16, 2013, 03:04:43 PM
You need to have you argon inlet on top of your condenser.
Any reason why?
Still prefer a mechanical stirrer!
Couldn't find one :(
Post by: discodermolide on March 16, 2013, 03:19:17 PM
[/quote]
LOL, just make sure the magnet is always stirring.
Post by: Messi on March 16, 2013, 08:55:50 PM
LOL, just make sure the magnet is always stirring.
Yup, I put two big magnets in my RBF, and put the stirrer on a super high setting :)
I see what you're trying to do, and it seems feasable. However, it can be done more efficiently:
You can "protect" the amine by forming the hydrobromide salt:
Then heat it with PBr3 to get the alkyl bromide.
Regarding this Dan, would the easiest way to do this be just to react my amine with HBr, in let's say either ether or THF as a solvent, and then without any purification just to add PBr3 to my solution and reflux? After these reactions, I purify my amine? Won't my amine be in salt form? Having it in free-base form is a better idea if I plan on doing another reaction with this amine, no?
Post by: orgopete on March 17, 2013, 02:47:23 AM
You need to have you argon inlet on top of your condenser.
Any reason why?
I prefer to use a condenser to reflux the solvent and not the argon inlet. The set up is virtually like having a big stopper on the side of the flask. If the reaction began refluxing, the vapors will push out the argon inlet.
Post by: Dan on March 18, 2013, 04:38:24 AM
Regarding this Dan, would the easiest way to do this be just to react my amine with HBr, in let's say either ether or THF as a solvent, and then without any purification just to add PBr3 to my solution and reflux? After these reactions, I purify my amine? Won't my amine be in salt form? Having it in free-base form is a better idea if I plan on doing another reaction with this amine, no?
Essentially yes. These procedures are published, just check the literature.
The free-base form of this compound is probably fairly unstable so I would isolate it as a salt and generate the free base for in situ in the next step.
Post by: Messi on March 18, 2013, 03:00:07 PM
Essentially yes. These procedures are published, just check the literature.
Yup, I got it! http://www.orgsyn.org/orgsyn/orgsyn/prepcontent.asp?prep=cv2p0358#Note169N2 (http://www.orgsyn.org/orgsyn/orgsyn/prepcontent.asp?prep=cv2p0358#Note169N2)
The free-base form of this compound is probably fairly unstable so I would isolate it as a salt and generate the free base for in situ in the next step.
Yup, I understand this. Thing is, wouldn't I want to run a column on my product after I brominate it before I do the next reaction?
Post by: discodermolide on March 18, 2013, 03:22:48 PM
Post by: Messi on March 18, 2013, 03:35:57 PM
Post by: discodermolide on March 18, 2013, 03:43:28 PM
Post by: Messi on March 18, 2013, 03:55:13 PM
Yes, I definitely isolated something. I see a yellow/red oil with a proline like smell to it.
Post by: discodermolide on March 18, 2013, 03:58:17 PM
Post by: Messi on March 18, 2013, 04:04:33 PM
You need a deuterated solvent for the H-NMR
Yup! By the way, do you agree with me that it is indeed odd that my product isn't soluble with chloroform and isn't showing up in the NMR?
Post by: discodermolide on March 18, 2013, 04:12:58 PM
What does the TLC look like?
Post by: Messi on March 18, 2013, 04:41:26 PM
Took my HNMR in deuterated methanol, thumbs up! Successful reaction! Now to add my bromine tonight :)
Post by: Messi on March 18, 2013, 09:49:48 PM
A mixture of (S)-amino alcohols 5 (0.15 mol) dissolved in 20% HBr aqueous solution (130 mL) was concentrated to dryness under reduced pressure. Owing to complete removal of water, the residue was dissolved in hot EtOH (40 mL) and the solvent was evaporated in vacuum to give amino alcohol hydrobromides. The obtained hydrobromide salts were then mixed with PBr3 (25.44 g, 0.093 mol) and refluxed for 10 min. After cooling, an excess of PBr3 was then removed by washing the mixture with Et2O several times and the residue was recrystallized from i-PrOH to afford 6a–e (over 86% yields).
I am trying to translate this procedure to layman terms. Does the above procedure translate to:
1) Dissolve amino alcohol in an HBr solution in an RBF
2) Add hot EtOH to RBF (Any reason why hot?)
3) Throw mixture on rotavap (How long?)
4) Add PBr3 to RBF and reflux for ten minutes
5) Throw RBF contents in a buchner funnel and aspirate via vacuum filtration
6) Collect crystal/salts and undergo next step in my reaction (Purification not necessary?)
Post by: discodermolide on March 18, 2013, 11:16:22 PM
The procedure tells you exactly what to do.
The solution of the amino alcohol in HBr/water was evaporated to dryness, then hot ethanol was added. The re-evaporated in vacuum. This is to remove all traces of water before adding PBr3. Ethanol and water form an azeotrope.
You evaporate until it is dry.
After the reaction with PBr3 you then cool and add ether and filter, wash the solid with ether several times. This removes excess PBr3.
You then dissolve that residue in iso-propanol and crystallise the product. This is the purification step.
Post by: Messi on March 18, 2013, 11:54:19 PM
Well you are not really a layman anymore, not after doing a LiAlH4 reaction :P
I beg to differ :) I still have a lot of learning to do! I have been doing a lot of searches on SciFinder to try learn quickly and efficiently!
Ethanol and water form an azeotrope.
Now I understand! This is ingenious! Awesome!
After the reaction with PBr3 you then cool and add ether and filter, wash the solid with ether several times. This removes excess PBr3.
When you say filter here, do you mean vacuum filtration or gravity filtration?
You then dissolve that residue in iso-propanol and crystallise the product. This is the purification step.
Easy enough, add my solid/salt to a warm solution of iso-propanol then let it cool. I will then add this cooled solution (hopefully I see crystals in my E-flask) to a Buchner funnel and purify it via vacuum filtration!
Side note: I plan on reacting this amine with another compound after. Do you recommend I do this reaction in situ? Also, I need the amine in its free base form when I do the reaction, so all I do is react the salt with NaOH then do my reaction?
Post by: discodermolide on March 19, 2013, 12:00:19 AM
For the next step: Now I don't know what it is.
But the free amine and the bromomethyl groups are not compatible.
So it may be worth thinking about mixing your next reactant with the HBr amine salt and then add a base such as triethylamine.
Post by: Messi on March 19, 2013, 12:10:28 AM
You usually use vacuum filtration.
Ok, nice, thanks!
For the next step: Now I don't know what it is.
But the free amine and the bromomethyl groups are not compatible.
So it may be worth thinking about mixing your next reactant with the HBr amine salt and then add a base such as triethylamine.
My reaction is where I react a thioketene with my amine. R2C=C=S + NHR2 This will create a thioamide. However, I need my amine in free-base form. Can I throw in my amine + base with my thioketene with the hope that the amine will undergo free base transformation and react with the thioketene? Thing is, I also use TFA for a proton source whenever I add my amine to my thioketene. I am skeptical of using triethylamine in this case because it might add to my thioketene.
Post by: discodermolide on March 19, 2013, 12:22:51 AM
If it has a precursor then you mix them together and add a base such as triethylamine or Hünig's base.
Triethylamine should not add to the thioketene.
Post by: Messi on March 19, 2013, 12:28:11 AM
Do you generate the thioketene in situ, from what?
Yes, I do. From 1-heptyne + nBuLi + sulfur I get my thioketene. Once I have this, I add an acid TFA and react it with my amine with the hope of getting a thioamide.
My final goal is to add LDA to this thioamide to generate an enolate with the hope that the enolate will attack the electrophilic carbon bonded to the bromine and create a heterocyclic system.
If it has a precursor then you mix them together and add a base such as triethylamine or Hünig's base.
Triethylamine should not add to the thioketene.
So you suggest I add 1-heptyne + nBuLi + sulfur + amine + triethylamine all together in one pot to start off? Would it be fine to forgo the addition of triethylamine and simply add two equivalents of nBuLi?
The free amine and the bromomethyl groups are not compatible.
Why is this? Intramolecular reaction? Won't the ring size be unfavorable?
Post by: discodermolide on March 19, 2013, 12:36:03 AM
I would pre-make the solution of thioketene and add it to a suspension of the amino alcohol.
Do you know if your thioketene is stable to another equivalent of BuLi (by the way TFA is used to quench the reactions you have been doing)
p.s. BuLi is quite nucleophillic and coule displace the bromine from your primary halide! Just so you are aware of this.
Post by: Messi on March 19, 2013, 12:59:13 AM
I would pre-make the solution of thioketene and add it to a suspension of the amino alcohol.
Pre-make the solution of thioketene with two equivalents of nBuLi?
Do you know if your thioketene is stable to another equivalent of BuLi
Yes, I believe it is.
p.s. BuLi is quite nucleophillic and could displace the bromine from your primary halide! Just so you are aware of this.
How about this work around:
Create my thioketene by reacting my 1-heptyne + 1 equivalence of nBuLi + sulfur then add my salt form of my amine + triethylamine then add my TFA. Would this work? Or would the triethylamine simply get neutralized by TFA?
But the free amine and the bromomethyl groups are not compatible.
Why is this exactly?
Post by: discodermolide on March 19, 2013, 01:28:27 AM
As I said the TFA is there for the work up, do not add it until the reaction is finished.
Amines are readily alkylated by alkyl halides.
If you make the free base of your amine it will react with the primary bromide. So you generate the free base in the presence of your other reaction partner, the thioketene. This should ensure the amine will react as desired.
Post by: Messi on March 19, 2013, 01:33:49 AM
When you say suspension, do you simply mean add my thioketene solution to my amine hydrobromide salt? Any reason why not vice-versa? Sorry, just me trying to understand what is going on more deeply :)
PS: Are you a professor/supervisor? You would be awesome to work for :)
Post by: discodermolide on March 19, 2013, 01:53:53 AM
Anyway:
It is easier to add the solution to a solid than a solid to a solution. AND you must get the free base of the amine. By adding the thioketene to the amine hydrobromide and then adding a base you will set the amine free and it should react with the thioketene.
As I said if you set the amine free first it will react with itself, before it can react with the thioketene.
Post by: Messi on March 19, 2013, 11:26:21 AM
Post by: discodermolide on March 19, 2013, 11:27:19 AM
Post by: Messi on March 19, 2013, 05:30:45 PM
I found this procedure:
A mixture of (S)-amino alcohols 5 (0.15 mol) dissolved in 20% HBr aqueous solution (130 mL) was concentrated to dryness under reduced pressure. Owing to complete removal of water, the residue was dissolved in hot EtOH (40 mL) and the solvent was evaporated in vacuum to give amino alcohol hydrobromides. The obtained hydrobromide salts were then mixed with PBr3 (25.44 g, 0.093 mol) and refluxed for 10 min. After cooling, an excess of PBr3 was then removed by washing the mixture with Et2O several times and the residue was recrystallized from i-PrOH to afford 6a–e (over 86% yields).
I am trying to translate this procedure to layman terms. Does the above procedure translate to:
1) Dissolve amino alcohol in an HBr solution in an RBF
2) Add hot EtOH to RBF (Any reason why hot?)
3) Throw mixture on rotavap (How long?)
4) Add PBr3 to RBF and reflux for ten minutes
5) Throw RBF contents in a buchner funnel and aspirate via vacuum filtration
6) Collect crystal/salts and undergo next step in my reaction (Purification not necessary?)
Great... I did my vacuum filtration.... and got no crystals... AWESOME... ???
Not sure what happened... I'm so confused.
Post by: discodermolide on March 19, 2013, 08:26:04 PM
In that procedure you posted what were the amino-alcohols 5?
Post by: Messi on March 19, 2013, 09:19:45 PM
Then evaporate the filtrate.
In that procedure you posted what were the amino-alcohols 5?
They included 5a-e which one of them was prolinol!
The solution of the amino alcohol in HBr/water was evaporated to dryness, then hot ethanol was added.
When you said was evaporated to dryness, how do you know when it is dry? I had rotovapped my solution for a good 15 minutes and still had all my solvent so decided to proceed to the next step. Maybe this was my mistake?
Also, I never saw one crystal. Everything was always liquid. Maybe I added too much solvent?
Post by: discodermolide on March 19, 2013, 09:24:35 PM
Did you apply a vacuum? It is more or less dry when the weight of the flask and contents is a constant weight.
So effectively you added the PBr3 to the ethanolic solution?
Post by: Messi on March 19, 2013, 09:36:41 PM
I then added warm ethanol and tried rotavapping again and nothing came off. I then added my PBr3 as per procedure.
I just ran another reduction of proline now, should be ready by tomorrow. I will have a second batch of prolinol. Hopefully this time my reaction will work...
Post by: discodermolide on March 19, 2013, 09:44:36 PM
The first evaporation is the removal of water. This takes time, a good vacuum and a warm water bath, 40°C or more.
Here it is important that you get almost all the water removed. The step with the ethanol evaporation removes the rest of the water. This is also important and gets it dry before adding the PBr3.
You have been lucky as PBr3 and water and or ethanol react violently!
You must take your time and do not rush things, think carefully, but don't rush.
Post by: Messi on March 19, 2013, 09:58:51 PM
You have been lucky as PBr3 and water and or ethanol react violently!
Yes, I agree. I didn't see any "violent" reaction. Where do you think I "messed" up however. This is quite odd. I did everything and literature told me to do and I still didn't get any crystals!
I thought I did indeed make my prolinol as per the NMR shown below...
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi45.tinypic.com%2F6f8lcw.jpg&hash=bb902c411fb5eaf694d401e568227caf3bd95e3d)
Post by: discodermolide on March 19, 2013, 10:10:24 PM
That's a shitty NMR, sorry, you need to get rid of the solvent signal at around 5ppm. Get the integration sorted out and make the peaks bigger so you can actually see them properly.
Post by: Messi on March 19, 2013, 11:27:52 PM
That's a shitty NMR, sorry, you need to get rid of the solvent signal at around 5ppm. Get the integration sorted out and make the peaks bigger so you can actually see them properly.
I agree. That signal around 5ppm was water.
Well you did not do everything as the literature said, you still had water left when you added PBr3 and the reaction mixture after the HBr addition was not dry!
So what should I see after I rotavap my solution of HBr with my amino-alcohol? Should I see no liquid, or very little liquid? Should I see my solid/salt?
The reason I am so confused is because prolinol has a boiling point of 74-76 °C at 2 mmHg while water has a boiling point of 100°C.
Post by: discodermolide on March 20, 2013, 12:06:31 AM
You won't be able to remove all the water which is why they add ethanol. You need to do that, you can even do it several times. Then you add the PBr3.
Why are you confused about the boiling points? Your compound will not vanish in the rotavap. the BPt. of water at 2mmHg is not 100°C but around 0°C. So a standard water aspirator will do the job for you.
Post by: Goala on March 20, 2013, 01:45:38 AM
Post by: discodermolide on March 20, 2013, 02:07:07 AM
Im only a 3rd yr student here. But it's odd that you are using a rotaryevaporator to evaporate water. For all the reactions I have done, I simply add a drying agent like magnesium sulphate. From what my lab instructors have told me, rotaryevaporating water takes a very very long time. I find it peculiar that the experimental procedure yoou are following states to do so.
Are you going to remove 130mL or more water with a drying agent? You will need kg of the stuff. I see absolutely no problem by rotavaping it off.
Post by: Goala on March 20, 2013, 02:17:50 AM
Are you going to remove 130mL or more water with a drying agent? You will need kg of the stuff. I see absolutely no problem by rotavaping it off.
Well, how long do you think it will take to rotaryevaporate it? I imagine it could take a good 35-45 minutes to rotaryevaporate 50-75 mL of water under 760 torr pressure. Am I correct in making this assumption?
Post by: discodermolide on March 20, 2013, 02:21:25 AM
Drying agents are only used to remove the last traces of water from a solvent not to remove large quantities.
Post by: Messi on March 20, 2013, 05:50:50 PM
I posted a picture of my "product" down below. It has been under the rotovap for two hours. I don't see crystals/solid like I thought I would... So, can I continue my reaction and add PBr3? Or should I let it on the rotovap overnight? I am unsure! I also posted a video below!
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi49.tinypic.com%2F2rf4sif.jpg&hash=fea10330ac31329a2872a71f3963e034a66838cb)
View My Video (http://tinypic.com/r/10hn19d/6)
I was tilting my RBF with my contents in it in this video.
Post by: discodermolide on March 20, 2013, 09:57:05 PM
If not, then do this let's say twice.
Post by: Messi on March 20, 2013, 10:11:26 PM
Has it been treated with the hot ethanol yet?
If not, then do this let's say twice.
Yes, it has been treated with hot ethanol twice!
Post by: discodermolide on March 20, 2013, 10:17:15 PM
Take a small part of your yellow gum? and do the next step. How much have you got 5 g or so? Try the next step on 0.5g and see what happens.
Post by: Messi on March 20, 2013, 10:42:19 PM
OK. It looks like you have quite a bit of it. Take a pasteur pipette and try and get rid of than oily liquid. Don't throw it away.
Take a small part of your yellow gum? and do the next step. How much have you got 5 g or so? Try the next step on 0.5g and see what happens.
I think my oily liquid is my yellow gum? I tried transfering a small portion of my oily liquid to a small rbf... a real hassle. Should I react my PBr3 with this oily liquid as a "test rxn"?
Shown below is my black oil.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi48.tinypic.com%2Ffc0f1v.jpg&hash=b3155a3b7c9c4a67dddedd7523e24f487a00a4f0)
Post by: discodermolide on March 20, 2013, 10:54:02 PM
I meant use the yellow solid for the test reaction.
Have you run NMR's yet?
Post by: Messi on March 20, 2013, 11:01:58 PM
Where is the other part? The yellow solid.
I meant use the yellow solid for the test reaction.
Have you run NMR's yet?
Unfortunately the other picture gave the illusion of there being a yellow solid. There is no yellow solid. It is just the color of the walls of the RBF after the oil dripped down the side of the walls. All there is in my RBF is a black oil that moves very slowly.
No, I haven't ran an NMR yet. I was following the procedure that I had showed you earlier. Technially after I add HBr, I should have a my salt in solid form... but I just have this gooey oil.
Post by: discodermolide on March 20, 2013, 11:04:25 PM
Try a test reaction on that dark red oil.
Have you run an NMR, what is the yield, assuming that's your product?
Post by: Messi on March 20, 2013, 11:15:04 PM
OK, that's clear now.
Try a test reaction on that dark red oil.
Have you run an NMR, what is the yield, assuming that's your product?
Yes, just did a test reaction... let's see what I get. Theoretically speaking, I should get some nice white crystals, no?
Yes, I had ran an NMR of prolinol, I got the spectra shown below.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi46.tinypic.com%2F1z2dpxs.jpg&hash=f907ee40c7e0323ff33901ff9da7ea6dbfb82fbd)
Post by: discodermolide on March 20, 2013, 11:21:00 PM
The prolinol looks ok. Did you run a NMR of the dark red oil? If not do so while you are waiting.
Post by: Messi on March 20, 2013, 11:55:14 PM
If you get nice white crystals from that dark red oil then I will be pleased.
No crystals what so ever.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi48.tinypic.com%2F351zsic.jpg&hash=599c6174c306378ef8ac85ff6d6d09a127ea04ad)
I just took NMR of my oil shown below:
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi46.tinypic.com%2F258cihf.jpg&hash=0a1399323e99cfe6f3903504312e40885591b7d1)
Post by: discodermolide on March 21, 2013, 12:14:23 AM
Post by: Messi on March 21, 2013, 12:17:50 AM
So things changed. Try and analyse both the spectra.
Well technically things should have changed, no? I add HBr so my amine gets protonated and maybe some HBr might replace my hydroxyl in prolinol (Br replaces OH).
This is so odd! Not sure what I should do now...
Post by: discodermolide on March 21, 2013, 12:21:32 AM
Try and purify a bit of your red oil. I think the method used iso-propanol?
Post by: Messi on March 21, 2013, 12:43:50 AM
That could have happened.
Try and purify a bit of your red oil. I think the method used iso-propanol?
The thing I'm confused about is that I thought whenever an amine is protonated with an acid like HBr it switches to salt form. I have no idea why this is not happening here!
Post by: discodermolide on March 21, 2013, 12:55:17 AM
Post by: Messi on March 21, 2013, 01:10:15 AM
It does form the HBr salt. Just try and crystallise it.
I read this somewhere:
One caveat that I noticed in my lab days long ago is that
hydrohalide salts will clump out in an oily mass if the
gas or the amine solution is the slightest bit wet.
Is this what is perhaps happening?
Post by: discodermolide on March 21, 2013, 01:15:15 AM
Add a bit of anhydrous sodium sulphate shake and filter it.
Remove the isopropanol and try the crystallisation again.
It does not look so red now, a nice yellow gum.
Remember do not add too much Iso-propanol for the crystallisation, start with a small quantity warm it up and add more until the solid dissolves, then cool it slowly.
Post by: Messi on March 21, 2013, 01:19:00 AM
That could be.
Add a bit of anhydrous sodium sulphate shake and filter it.
Remove the isopropanol and try the crystallisation again.
It does not look so red now, a nice yellow gum.
Remember do not add too much Iso-propanol for the crystallisation, start with a small quantity warm it up and add more until the solid dissolves, then cool it slowly.
Should I do this before adding my PBr3?
Post by: discodermolide on March 21, 2013, 01:21:14 AM
How is the test reaction looking?
Post by: Messi on March 21, 2013, 01:31:17 AM
Yes.
How is the test reaction looking?
Unfortunately not very well. I am not sure if I am mixing up my steps or what is going on. I don't know what to say...
Post by: discodermolide on March 21, 2013, 01:33:40 AM
Yes.
How is the test reaction looking?
Unfortunately not very well. I am not sure if I am mixing up my steps or what is going on. I don't know what to say...
What is happening?
Post by: Messi on March 21, 2013, 02:14:51 AM
Post by: discodermolide on March 21, 2013, 02:26:14 AM
Then let's think about the PBr3 step.
Post by: discodermolide on March 21, 2013, 02:37:23 AM
http://sdbs.riodb.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi (http://sdbs.riodb.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi)
search under pyrrolidinemethanol
This is not the HBr salt.
Post by: Messi on March 21, 2013, 07:44:38 PM
I have my usual liquid above it swirling patiently.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi45.tinypic.com%2F2f08zyv.jpg&hash=3d95d3e71080c9e9552f3fcf66a7d88ba0e01c57)
Post by: Corribus on March 21, 2013, 08:50:15 PM
Post by: discodermolide on March 21, 2013, 10:02:43 PM
Post by: Messi on March 21, 2013, 10:15:42 PM
Did you add a base to make the free amine?
Yes, added TEA.
Post by: discodermolide on March 21, 2013, 10:35:22 PM
Post by: Messi on March 21, 2013, 10:37:07 PM
Did the TLC of your thioketene RM show a new product?
I took a 5:1 Hex:EtOAc TLC and didn't see anything. I think I need a more polar eluent perhaps. Theoretically speaking, the compound I should have made was a thioamide with a branched bromine.
Post by: discodermolide on March 21, 2013, 10:51:20 PM
Post by: Messi on March 22, 2013, 08:00:36 PM
Took NMR of final product. It was certainly not the product I was looking for I don't think. NMR shown below. This sucks!
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi50.tinypic.com%2F23hsr9j.jpg&hash=67a6885ff2342ffc7990943ab97667ec7b18a5f7)
Post by: discodermolide on March 22, 2013, 11:28:59 PM
Is this a single spot on TLC?
Does it correspond to anything you have seen in the past in this series of thioketene experiments?
Can you get a MS done?
It may be worth thinking about using protection to do this chemistry instead of trying the "quick way"?
Post by: Messi on March 23, 2013, 01:04:30 AM
Sorry, but another useless NMR. Too much chloroform in it, too much TMS. The phase needs correction, as do the integrals. Sort that out and expand the region between 5-0.5 ppm, in the vertical as well.
Good idea, will do that.
Is this a single spot on TLC?
Does it correspond to anything you have seen in the past in this series of thioketene experiments?
I have a few spots on my TLC. It doesn't particularly correspond to anything I have seen in the past. When using pyrrollidine as an amine and getting the thioamide, the hydrogens of the carbons adjacent to nitrogen had a chemical shift of 3-4 which doesn't seem to be the case here...
It may be worth thinking about using protection to do this chemistry instead of trying the "quick way"?
Yes, good idea. Do you know of any reason why my reaction may not be working however?
Post by: discodermolide on March 23, 2013, 01:27:33 AM
I suggested you add the thioketene to the amine hydrobromide and then add triethylamine, you didn't do that.
Most importantly you need to make sure that the amine hydrobromide salt is what you think it is.
p.s. the quick and dirty routes don't usually work very well. Take each step and make sure it is what it is supposed to be and pure before using it in the next step.
Post by: Messi on March 23, 2013, 01:43:24 AM
I suggested you add the thioketene to the amine hydrobromide and then add triethylamine, you didn't do that.
I did do that :)
p.s. the quick and dirty routes don't usually work very well. Take each step and make sure it is what it is supposed to be and pure before using it in the next step.
Columns are so painful!
Post by: discodermolide on March 23, 2013, 01:50:46 AM
So you added the salt to the thioketene.
Columns are a part of organic chemistry. If you can crystallise something fine, if not you need to chromatograph it.
It is vital to have pure compounds especially at the beginning of a new synthetic sequence. Later on when you gain experience with the reaction(s) you get to know what needs to be pure and what doesn't.
Post by: Messi on March 23, 2013, 02:38:34 AM
So you added the salt to the thioketene.
I made a mistake earlier when I said what I did. I did indeed add my thioketene to the salt :)
By the way, random question: I made a an amine that was on my triple bond (heptyne), and I did the reaction to trap the thioketene with my amine to create the thioamide but it didn't work. Usually these cyclization reactions with a ketene (C=C=O) are done at temperatures of ~60°C, however to create a thioketene and to react it with an amine it must be done at -78°C. Could this be a reason why when I try to cyclize and create a thioamide it isn't working?
Post by: discodermolide on March 23, 2013, 02:46:03 AM
Question, why does the thioketene generation have to be done at -78°C, do you know this for fact? Have you tried it at a higher temperature? I would find out exactly what temperature you can use for this thioketene generation and trapping with an amine.
Post by: Messi on March 23, 2013, 03:25:04 AM
It could be.
Question, why does the thioketene generation have to be done at -78°C, do you know this for fact? Have you tried it at a higher temperature? I would find out exactly what temperature you can use for this thioketene generation and trapping with an amine.
I have tried it at -78°C and have had best results with it (better yield, less side reactions, cleaner). I also tried it at 0°C and the reaction did work, but with a much lower yield. A paper I found, said to do the reaction at -78°C. I guess I should try the reaction tomorrow at 50°C with the hope that it doesn't work so my "hypothesis" is correct? ;D
I believe ΔG=ΔH-TΔS has something to do it, no?
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi50.tinypic.com%2F5koubs.jpg&hash=83565ade21eea527d96da47b81a949540bfcd709)
Post by: discodermolide on March 23, 2013, 03:51:16 AM
You need to find the highest temperature that gives you the best yield and cleanest product.
Post by: Messi on March 23, 2013, 03:55:03 AM
I hope you mean -50°C. Also try at -25°C, -10°C.
You need to find the highest temperature that gives you the best yield and cleanest product.
Agreed of course!
Well, do you think my rationale of using ΔG=ΔH-TΔS as an argument is valid as to why I am not seeing a reaction? Come to think about it... I'm not sure if it is a valid argument or not.
When we do cyclization reactions, how come we always see heat (Δ) as one of the necessities, and we rarely see cyclization reactions under cold temperatures?
Post by: discodermolide on March 23, 2013, 04:07:10 AM
There are plenty of cyclisations that go at lower temperatures.
Have a look here for some guidelines.
http://en.wikipedia.org/wiki/Baldwin's_rules (http://en.wikipedia.org/wiki/Baldwin's_rules)
Post by: Messi on March 23, 2013, 04:12:17 AM
Have a look here for some guidelines.
http://en.wikipedia.org/wiki/Baldwin's_rules (http://en.wikipedia.org/wiki/Baldwin's_rules)
Thanks! I just had a read, interesting.
Usually you need to heat it to overcome any ring strain energy that may be a barrier to cyclisation.
Hopefully in my case, the reason my reaction is not working because temperature isn't high enough :) What do you think, am I right? Is it logical? I am going to bring it up to my supervisor tomorrow and see what he says... :)
Post by: discodermolide on March 23, 2013, 04:14:16 AM
Post by: Messi on April 01, 2013, 02:55:04 PM
Sure ask him his opinion.
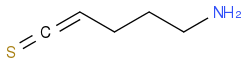
For the reaction below to take place, would heat be a necessity?
Post by: discodermolide on April 01, 2013, 03:28:51 PM
Post by: Messi on April 01, 2013, 04:04:29 PM
Probably not. Heat it and you will end up with a series of many polymeric amines. It may not even give much cyclic product.
So my earlier rationale regarding cyclization of the thiolactam shown below is false? I don't need heat to induce cyclization?
Post by: discodermolide on April 02, 2013, 01:22:28 AM
Post by: Messi on April 02, 2013, 01:59:03 AM
You cannot compare an alkyl halide and a thioketene in reactivity. The thioketene should be more reactive towards nucleophiles.
So you are saying it most likely does not need heat to cyclize? Heat was my basis of argument at to why cyclization was not occurring. Am I wrong!?
Post by: discodermolide on April 02, 2013, 02:06:56 AM
Post by: Messi on April 02, 2013, 02:10:40 AM
You are doing the reaction at -75°C, if I remember correctly. Let it warm to 0°C and see what happens, that's heating the reaction!
I did do this, and the reaction didn't work! No cyclization.
Does it make sense that it isn't working though? My whole paper is based on the argument that heat is needed for the reaction to take place. Am I actually wrong? Is my argument weak? I want an honest answer :)
Post by: discodermolide on April 02, 2013, 02:17:58 AM
a) perhaps you did not form your amine.
b) perhaps the thioketene did not form
c) try heating it to 70°C to confirm your theory
d) Check the structures of all your intermediates, H-NMR, MS etc on pure materials.
e) Try trapping the thioketene with something you know works.
Post by: Messi on April 02, 2013, 02:23:30 AM
It does not make sense.
What does not make sense? My argument, or my reaction not working? Or both? :-[
c) try heating it to 70°C to confirm your theory
My thioketene does not form at 70°C. I tried it.
Post by: discodermolide on April 02, 2013, 02:31:24 AM
This may suggest that you do not have the structures you think you have.
Post by: Messi on April 02, 2013, 02:37:11 AM
1) Thioketene generation occurs best at -78°C. So once I create my thioketene I trap it with an amine to create the corresponding thioamide.
2) I would like to create a thiolactam, so I undergo the reaction intramolecularly with an amine functionality at -78°C and find that my thiolactam is not produced.
3) I realize that this might be a thermodynamic problem. Most of the time heat is required for cyclization. (NOT SURE IF THIS IS VALID POINT)
4) I decide to do the cyclization reaction at 70°C and there is no reaction.
5) I decide to go back to step 1) and generate a thioketene at 70°C and react it with an amine to create a thioamide... no reaction.
6) My final argument is that in order to create a thiolactam, heat is required, and because thioketenes are best generated at -78°C, the creation of a thiolactam is not possible in this way. A different method must be taken where the generation of a thioketene be done at higher temperatures.
Thoughts on this argument?
Post by: discodermolide on April 02, 2013, 02:51:39 AM
If that does not produce the lactam then you can say a different method must be employed to generate thioketenes when they are going to be used to form lactams.
Post by: Messi on April 02, 2013, 02:55:02 AM
OK, you know the reaction works with an amine to give the thioamide. Perhaps the aniline was not the best choice for this reaction. why not use the normal amine without the aromatic ring.
Yes, I've tried hexylamine as well and no luck! Oh, the joys of science!
I just hope my heat argument holds up in court... or my supervisor will kill me :( As long as you think my argument is reasonable... I will be able to sleep well tonight. If not... well... :'(
Post by: discodermolide on April 02, 2013, 02:57:09 AM
Post by: Messi on April 02, 2013, 03:02:16 AM
In that case you can use your argument that thioketene to thiolactam cannot be done this way.
Thank you discodermolide for all the help; I truly appreciate it. Hopefully all ends well, and my supervisor does not think I am a doofus because I am using heat as an argument. Also, I hope I don't get a poor mark because my research was unsuccessful and my arguments flawed. Only time will tell, and I thank you discodermolide for all the help. God bless you.
Post by: discodermolide on April 02, 2013, 03:03:59 AM
Post by: orgopete on April 02, 2013, 09:16:36 AM
1) Thioketene generation occurs best at -78°C.
Thoughts on this argument?
Occurs best? This is the step I am most skeptical of. I know the reaction to prepare a thioketene was performed, but are you sure you have a thioketene?
Post by: Messi on April 02, 2013, 04:18:23 PM
Occurs best? This is the step I am most skeptical of. I know the reaction to prepare a thioketene was performed, but are you sure you have a thioketene?
Yes, from literature, forming a thioketene is done in such a matter. I have confirmed with the trapping of an amine that I have indeed formed the thioketene. NMR data confirms this.
Post by: orgopete on April 04, 2013, 09:05:42 AM
But is my argument reasonable? I am very curious what you think as your knowledge of organic chemistry (in my eyes) is AMAZING!
1) Thioketene generation occurs best at -78°C. So once I create my thioketene I trap it with an amine to create the corresponding thioamide.
2) I would like to create a thiolactam, so I undergo the reaction intramolecularly with an amine functionality at -78°C and find that my thiolactam is not produced.
3) I realize that this might be a thermodynamic problem. Most of the time heat is required for cyclization. (NOT SURE IF THIS IS VALID POINT)
4) I decide to do the cyclization reaction at 70°C and there is no reaction.
5) I decide to go back to step 1) and generate a thioketene at 70°C and react it with an amine to create a thioamide... no reaction.
6) My final argument is that in order to create a thiolactam, heat is required, and because thioketenes are best generated at -78°C, the creation of a thiolactam is not possible in this way. A different method must be taken where the generation of a thioketene be done at higher temperatures.
Thoughts on this argument?
I don't know what was or wasn't done. If you know a thioketene reacts with an amine to give a thioamide AND the above sequence of reactions does not give a thioamide, from my general knowledge and examining what appears to have been done, I was skeptical of the thioketene formation.
I've done some of these sulfur reactions and have found they can be very messy. Some of the prior descriptions appeared as though there was doubt about the thioketene, but the reaction was carried forward, but to failure. For me, ths is a red flag.
Post by: Messi on April 06, 2013, 09:21:11 PM
A problem may also be that not only the acetylide, but also the amino N may break up the S8 rings and possibly give some weird species with S-N bonds. So intermediate protection of the N may be helpful, e. g. bei silylation.
Maybe this is the solution! :D
Post by: Messi on April 11, 2013, 08:25:36 PM