Hey all, just reading a paper by Knochel (
dx.doi.org/10.1002/asia.200700099) and am interested in using his procedure to make a boronic ester from a MgCl.LiCl grignard species, however I am having a bit of difficulty working my head around this step:
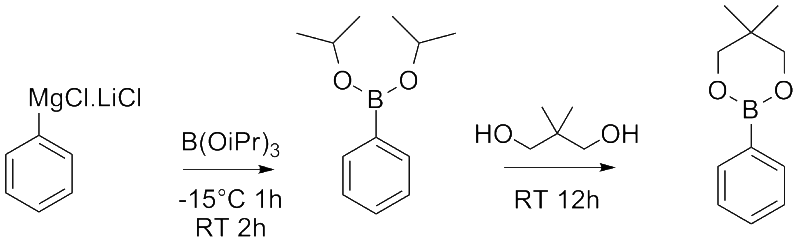
So, the grignard reagent is reacted with the electrophilic triisopropyl borate, standard stuff, but then neopentyl glycol is added, stirred at rt for 12 h and it transesterifys? What is going on here? 6mmol of B(OiPr)
3 is used vs 6.25mmol of neopentyl glycol, so its barely in excess, I would have though hardly enough to drive equilibrium to the neopentyl ester. There isn't any heating or use of a dean stark or anything like that, so what is happening to what I would have thought is the relatively stable isopropyl boronic ester?
I'm only interested in the boronic acid so was planning to hydrolyse after the isopropyl borate addition + stirring overnight. Are neopentyl boronate esters much more stable or reactive? If not, why does he not just hydrolyse instead of converting to the neopentyl ester?
Maybe its just a friday evening and my brain is mushy, but ideas anyone?