Hi.
I am wondering when should we use hybridized orbital?
For PH3, we don't use hybridized orbital because it could be described by the valence shell method.
p: [Ne] 3s:
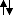
3p:



.
And we have 3 up. so the 3 extra electrons from hydrogen could fit into there. Therefore we don't need hybridized orbital.
But when do we need it?