The first compound doesn't have stereoisomers, it only has two diastereomers, because it is meso compound.
Many misunderstandings here:
1. Diastereoisomers are a type of stereoisomer. Enantiomers are another type of stereoisomer.
2. There are three stereoisomers of 1,2-dichlorocyclobutane (one
meso compound and a pair of enantiomers)
Try to rotate the molecule yourself and you will see that (1S,2R) and (1R,2S) are exactly the same.
As sjb says, IUPAC conventions assign
R to the lower locant in situations like this.
- In a cyclic compound such as this,
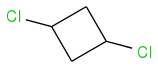
There are no stereocenters - correct? So you cannot use R or S notation?
There are pseudoasymmetric centres, and you can assign pseudoasymmetric descriptors
r/
s (note the lower case). It is quite a complicated assignment for cyclic structures such as these, and was briefly discussed previously
here for 1,4-dihydroxycyclohexane (the symmetry is analogous to this case). It is easier to use
cis/
trans.