Then you need to have a large excess of that unstable reagent say at -15 to -10°C and add your other reactant to it. Try and make the reaction addition controlled. Check it out on a small (TLC) scale for the exact conditions first.
I use the compounds in the ratio 1:2 eq, there is 1 eq of that unstable reagent and I add the 2 eq of stable substrate dropwise over a period of 2 hours (it worked of the other substrate, the reaction was done within 1 hour and the unstable reagent does not decompose), but this one should be left overnight. It's a bit confusing. And when it comes to TLC, both the product and unstable reagent are triflates, and they stick to the baseline of the TLC plate.
Not being elegant
If you have a freezer that can go to -30°C
Put reaction mixture on top of stirrer
put stirrer cord outside freezer
close door on cord so that grommet around door is deformed around said cord
Duct tape door close
Duct tape around the door as to seal it (if truly needed )
[I have a Tshirt that says I can most anything with Duct tape]
Do not do this with a freezer than may have food inside it during or after process
Regular household freezers are kept just below zero °C, so you may not have a freezer that goes low enough.
You said I did not have to be elegant.
Yep. I was thinkig about the same thing, and this not elegant, it's hilarious. We have fridges that cool down to -30°, I'm only affraid that the stirrers are a bit too big and I don't know if they will get damaged by cold. But it might work.
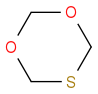
Exactly. Yet there is no entry in the SciFinder or Merck Index. I really don't know what to do or where to check. The last thing would be to contact the producer. However, even if they say that the product is discontinued, I'm still in trouble because I need a proof that this product actually exists, I mean, how I can't find it in the databases??