If in a ketone, two different enol forms are possible, eg:
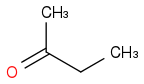
can give both
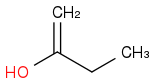
and
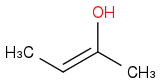
, which is major product in acidic medium?
I think the elimination of H should follow saytzeff rule, IE

should be in the majority, as abstraction of that H by acid ought to be easier. Would I be correct?