So it's just your basic run-of-the-mill add a base to a compound and attach another substituent to it. Or it should be anyway.
I've used methanol as solvent, added my base, potassium tert-butoxide, and allowed it to equilibriate for 10-15 minutes. Then I added the 2,4-pentanedione
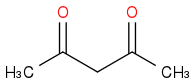
which was left again for 5 minutes followed by the addition of 4-nitrobenzyl bromide
=O)C=C1](https://www.chemicalforums.com/SMILES/e88eade90607e89d2292.png)
The mixture was then left stirring overnight and the solvent then removed via rotary evaporation. The product was analysed with NMR in CDCl
3 and it seems to have not attached in the centre but at the end like so
=O)C=C1)=O)=O](https://www.chemicalforums.com/SMILES/0a727624c899eb7c55cd.png)
Why has this happened? I added a 1:1 base mixture and surely the hydrogens on the 3- postion should be more acidic?
To make matters worse I'm struggling to recreate my results (basically no reaction occurs on some attempts).
Any suggestions as to why it reacts like it does or why I'm struggling to recreate my results would be much appreciated.
