My book lists the following reaction:
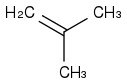
+Br
2, all in a methanol solvent

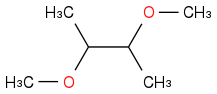
I'm not sure I understand the product. Wouldn't
![CC([Br])C(OC)C](https://www.chemicalforums.com/SMILES/979a854af1519a98d9c9.png)
be a more likely product? If there are no acids involved, I don't see that methanol could act as an electrophile, so one of the bromine must be an electrophile. Then, either the bromide ion or methanol could act as a nucleophile for the newly formed R-bromonium complex, with methanol being more likely as its present in larger concentrations.