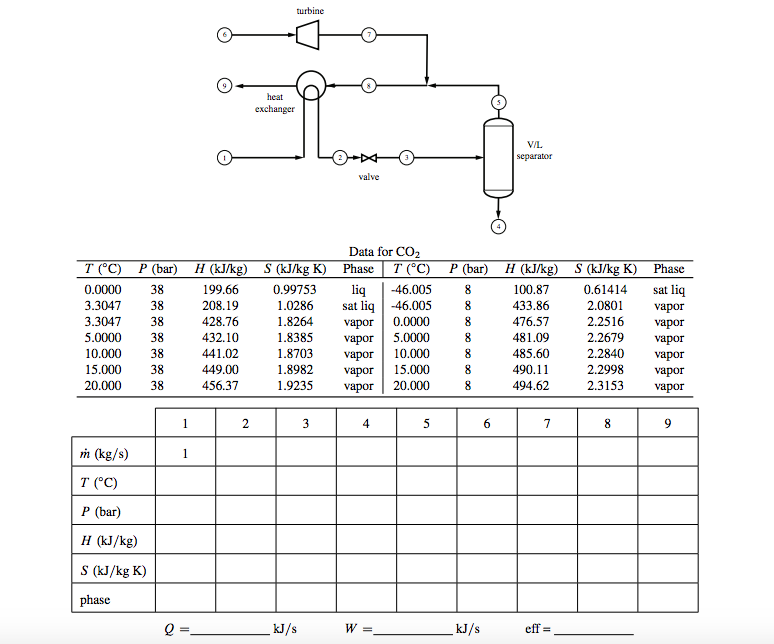
Chemical Engineering Question:
The process below is part of a system that liquefies carbon dioxide (all streams are CO2). Stream 1 is at 20 degree C, 38 bar, passes through the heat exchanger where it becomes saturated liquid (stream 2) and is then throttled to 8 bar (stream 3) to produce a vapor-liquid mixture. Stream 3 is separated in a vapor/liquid separator, with all vapor exiting in stream 5 and all liquid as stream 4. Stream 6 is at 20 degree C, 38 bar and expands in a turbine. Stream 7, which contains 5% liquid, is mixed with stream 5. The resulting stream 8 is passed through the heat exchanger and exits at 20 degree C. Use the data given below and a basis of 1 mol/s in stream 1 to do the following:
a) Obtain the thermodynamic properties (T , P, H, S, phase) of all streams. If the state is vapor-liquid mixture, instead of phase report the liquid fraction.
b) Calculate all molar flow rates.
c) Calculate the work in the turbine.
d) Calculate the efficiency of the turbine.
e) Calculate the amount of heat that is exchanged between the two streams in the heat exchanger.
Summarize your results in the table below but you must show your work.