There's a lot here, but its all over the place. Let's try to work through it all.
First off, you've posted in High School Chemistry, but some concepts are more advanced, but you seem to missing some basics. Which is fine, we're glad to help anyone, but having to reteach you the basics, while covering the advanced, is more work for us.
Re: Strong Oxidation of Carbon-Carbon Double Bond
This title has almost no bearing at all one the question setup or content. So. Again. Not a problem, really. But still, extra work.
Hello everyone, recently under the Chapter of Alkenes, I've learnt that upon strong oxidation of C=C bond with hot,acidic KMnO4, the terminal carbon bonded by a C=C bond will be oxidised into carbonic acid, and the final oxidised product will be CO2 and H2O.
OK. That's a good reaction to learn. There's also this one:
Na
2CO
3 + 2 H
+ 
2 Na
+ + H
2CO
3 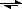
CO
2 + H
2O +2 Na
+So we could have just worked with that reaction, given the next questions you ask.
Though I understand that H2CO3 can dissociate,
Yes it can:
https://en.wikipedia.org/wiki/Carbonic_acid#Acidity_of_carbonic_acidThat reference introduces a number of concepts you're ignoring. The pKa is low, and is also affected by pressure and temperature. Also, dissociation of carbonic acid give H+, not the CO
2 plus water. Just letting you know we're getting muddled with the definitions here.
I was just wondering how is it possible for a slightly soluble product like H2CO3(aq) to form a gaseous CO2?
This is a decomposition reaction. Some things decompose. Some things, called acids, dissociate into H+. And some ionic salts dissociate into ions, in water. And some do more than one, at once, or so rapidly we only see one. You can ask again, if this bit is still confusing, but I don't know how to say that clearer.