I'm trying to determine which is the stronger/weaker acid/base,
CO
3-2 + H
2O
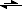
HCO
3- + OH
-the arrow pointing left is longer than the arrow pointing right, showing I think that the reaction is stronger in one direction, but other than the arrow what makes HCO
3- the stronger acid and OH
- the stronger base,
I really appreciate your help thanks