I tried the following reaction but didn't get any product, HNMR showed no reaction at all.I have found some examples on Reaxys, and tried the same contidions (DCM 25℃), but can't work.I also find some reaction like it (on reaxys) and use DMAP or Et
3N as catalyst but got nothing.
I also tried heating or ice bathing conditions, but it didn't work either.
I doubt that they can react and that the product can be stable at room temperature. I also want to know the mechanism in this reaction.
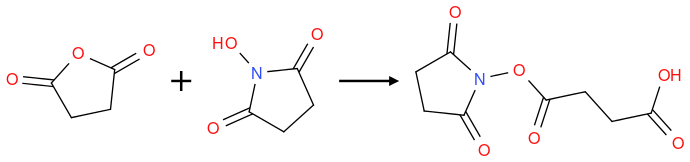
nmr of products: 1H NMR (300 MHz, DMSO) δ 10.53(s, 1H ), 3.32(s water), 2.90(s, 4H), 2.59(s, 4H). The eq. is NHS:SA = 1:1.
I am a newr in this forum and this is my first topic, i have read the form rules, but i'm not sure if there something wrong . Thank you all for your responses.