Post by: Lynda92 on April 15, 2015, 11:06:56 AM
I'm in doubt as to whether the phosphonium ylide attached below is classified as stabilised and therefore should give E-alkene selectivity in a Wittig reaction.
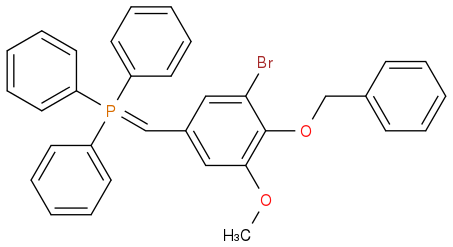
Post by: Dan on April 15, 2015, 11:47:04 AM
My gut feeling would be to the unstabilised side, but I haven't looked it up.
Post by: sjb on April 15, 2015, 12:33:35 PM