Post by: Lynda92 on January 27, 2015, 05:50:12 PM
I need help on calculating the volume of oxygen that is required to oxidise a dihydroxystillbene molecule (mass= 35mg, MW=430.09g/mol) into a diquinone methide molecule
So far I have calculated that 35 mg of dihydroxystillbene is equal to 0.082 mmol
A dihydroxystillbene molecule has 2 hydroxy groups on it, so theoretically for 1 mol of a dihydroxystillbene to be oxidised 2 mol of oxygen is required
So for 0.082 mmol of dihydroxystillbene to be oxidised, 0.164 mmol of oxygen is required
then using the ideal gas equation PV=nRT
V= 0.000164mol x8.314 JK-1mol-1 x 298K/ 101.325Pa
V = 0.00401 m^3
Is this correct???
Post by: billnotgatez on January 27, 2015, 06:13:02 PM
8.3144621 J K−1 mol−1
is the same as
8.3144621 m3 Pa K−1 mol−1
So the number 8.314 works
Post by: Borek on January 27, 2015, 06:19:18 PM
V = 0.00401 m^3
Is this correct???
You are on the right track, but your units are off.
Pressure should be not 101.3 Pa, but 101.3 kPa.
Post by: Lynda92 on January 27, 2015, 06:29:31 PM
V = 0.00401 m^3
Is this correct???
You are on the right track, but your units are off.
Pressure should be not 101.3 Pa, but 101.3 kPa.
thanks very much for spotting my mistake. so the answer is 4cm^3 of oxygen.
i have another question if you don't mind answering: since oxygen is only 20% component of air would i need 20cm^3 of air to achieved this reaction?
Post by: Borek on January 27, 2015, 06:34:51 PM
Please remember this is approximate, we have completely ignored humidity. It shouldn't change the answer by more than just a few tenths of a milliliter though.
Post by: Lynda92 on January 27, 2015, 06:46:09 PM
Yes, something like 20 mL of air should contain enough oxygen.
Please remember this is approximate, we have completely ignored humidity. It shouldn't change the answer by more than just a few tenths of a milliliter though.
thanks. that's a good point to make but no problem as i am only looking for an approximate value
Post by: mjc123 on January 28, 2015, 04:51:13 AM
Quote
A dihydroxystillbene molecule has 2 hydroxy groups on it, so theoretically for 1 mol of a dihydroxystillbene to be oxidised 2 mol of oxygen is requiredIs this true? Can you write a balanced equation for the reaction?
Post by: billnotgatez on January 28, 2015, 10:58:04 AM
From WIKI
http://en.wikipedia.org/wiki/3,4%E2%80%B2-Dihydroxystilbene
3,4'-Dihydroxystilbene
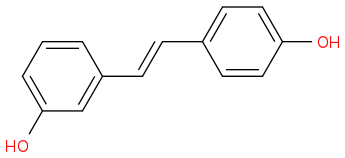
Molecular formula C14H12O2
Molar mass 212.24 g/mol
Post by: mjc123 on January 28, 2015, 12:28:07 PM
Post by: Lynda92 on January 28, 2015, 03:44:35 PM
Is this the stuff we are talking about
From WIKI
http://en.wikipedia.org/wiki/3,4%E2%80%B2-Dihydroxystilbene
3,4'-Dihydroxystilbene
Molecular formula C14H12O2
Molar mass 212.24 g/mol
It would have to be 4,4' to form a diquinone methide.
Yes you are correct that is the structure of dihydroxystillbene but what I meant to say in my original question was a 'dihydroxystillbene analogue with MW of 430'. Apologies for this confusion.
Yes you are correct it must be 4,4' dihydroxy in order to form a diquinone methide
Post by: Lynda92 on January 28, 2015, 03:57:53 PM
QuoteA dihydroxystillbene molecule has 2 hydroxy groups on it, so theoretically for 1 mol of a dihydroxystillbene to be oxidised 2 mol of oxygen is requiredIs this true? Can you write a balanced equation for the reaction?
I thought for 2 hydroxy groups i would need 2 molecules of oxygen to carry out a one electron oxidation reaction on each hydroxy group to form a diradical intermediate which then forms a diquinone methide (this occurs after the hydroxy groups are deprotonated, say in water). Am i incorrect?
Post by: mjc123 on January 29, 2015, 05:53:05 AM
I ask again, can you write a balanced equation for the reaction?