Post by: cookiecrumbzz on April 19, 2014, 02:31:26 AM
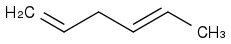
For the double bond between the first and second carbon, we have 2 alpha H's (i.e., the H atoms on the third carbon). For the double bond between the fourth and fifth carbon, we've got 5 alpha H's (i.e., 2 from the third carbon and 3 from the sixth carbon). How can I predict the number of ways in which hyperconjugation can occur?
The two H's on the third carbon serve as alpha H's for both the double bonds, which confuses me all the more when it comes to counting the possibilities.