Post by: Ter on December 12, 2012, 11:22:47 AM
This question was where we had to determine which molecule will have the smallest overall dipole. I understand why this molecule ( I'm going to describe it as the file was too big to upload..)
C-C double bond in the middle.
Left hand carbon has Cl atom at the top bonded to it , and CH3 molecule at the bottom.
Right hand carbon has Cl atom at the bottom bonded to it , and CH3 molecule at the top.
was the answer over the others, but I don't understand how this molecule can be symmetrical. If I were to fold from left to right, it won't be symmetrical for the CH3 will meet with the Cl and etc. If I fold along the double bond, it won't be symmetrical either! And from what I know it is impossible to rotate about the double bond, so the CH3 and Cl atoms will stay where they are won't they. So they will be fixed, and unsymmetrical, but it is symmetrical and non-polar, can someone please explain to me how?
Thank you!
Thank you!
Thank you!
Post by: curiouscat on December 12, 2012, 12:52:40 PM
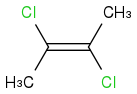
This?
Post by: Ter on December 13, 2012, 04:29:53 AM
Post by: Hunter2 on December 13, 2012, 05:05:40 AM