Post by: thumper101 on February 23, 2013, 08:01:00 PM
- In a cyclic compound such as this,

The first stereocenter is R and the second is S. Because you can number the cycle in any direction, is there any difference between (1R,2S) - 1,2 - dicholorobutane and (1S,2R)?
- In a cyclic compound such as this,
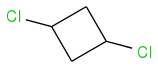
There are no stereocenters - correct? So you cannot use R or S notation?
Thank you!
Post by: CrazyAssasin on February 24, 2013, 01:48:11 AM
While speaking about the second question, yes, you are right, there are no stereocenters, because both carbons with chlorines have two identical substituents.
Post by: sjb on February 24, 2013, 05:26:27 AM
The first compound doesn't have stereoisomers, it only has two diastereomers, because it is meso compound. Try to rotate the molecule yourself and you will see that (1S,2R) and (1R,2S) are exactly the same. You should call two different diastereomers cis and trans.
While speaking about the second question, yes, you are right, there are no stereocenters, because both carbons with chlorines have two identical substituents.
Not quite, technically speaking you should still call the first (1R, 2S), R comes before S in naming.
Post by: Dan on February 24, 2013, 06:50:59 AM
The first compound doesn't have stereoisomers, it only has two diastereomers, because it is meso compound.
Many misunderstandings here:
1. Diastereoisomers are a type of stereoisomer. Enantiomers are another type of stereoisomer.
2. There are three stereoisomers of 1,2-dichlorocyclobutane (one meso compound and a pair of enantiomers)
Quote
Try to rotate the molecule yourself and you will see that (1S,2R) and (1R,2S) are exactly the same.
As sjb says, IUPAC conventions assign R to the lower locant in situations like this.
Quote from: thumper101
- In a cyclic compound such as this,
There are no stereocenters - correct? So you cannot use R or S notation?
There are pseudoasymmetric centres, and you can assign pseudoasymmetric descriptors r/s (note the lower case). It is quite a complicated assignment for cyclic structures such as these, and was briefly discussed previously here (http://www.chemicalforums.com/index.php?topic=65098.msg234922#msg234922) for 1,4-dihydroxycyclohexane (the symmetry is analogous to this case). It is easier to use cis/trans.