Post by: The Guy on December 04, 2013, 09:13:51 PM
I am thinking of PCC and I am afraid that it will oxidize the double bond too, to produce epoxied or alcohol.
Is the double bond sensitive to slightly acidic reagent?
Thank you
Post by: Archer on December 04, 2013, 09:29:48 PM
Look up Corey & Suggs original paper for PCC oxidations for more detail.
Post by: discodermolide on December 04, 2013, 10:22:51 PM
Post by: Archer on December 04, 2013, 10:34:02 PM
I prefer either TEMPO/Bleach or a Swern oxidation.
I agree, PCC is very messy, in my experience Swern is superior provided you take precautions to keep the smell in the fume hood. I have never run a TEMPO/Bleach reaction so couldn't comment on it.
Post by: spirochete on December 04, 2013, 11:03:59 PM
Post by: Archer on December 04, 2013, 11:16:27 PM
A harder question would be how to selectively oxidize a C=C pi bond in the presence of a primary or secondary alcohol? I am now genuinely curious about the best way to do this. Do things like OsO4 selectively dihydroxlate a pi bond or do they do funny side reactions with alcohols?
Catalytic OsO4 dihydroxylation reactions of olefins are carried out in acetone with t-butanol (used to disolve and store the highly toxic OsO4) and tBuOOH to regenerate the OsO4 so alcohols are unaffected.
Post by: spirochete on December 04, 2013, 11:47:02 PM
A harder question would be how to selectively oxidize a C=C pi bond in the presence of a primary or secondary alcohol? I am now genuinely curious about the best way to do this. Do things like OsO4 selectively dihydroxlate a pi bond or do they do funny side reactions with alcohols?
Catalytic OsO4 dihydroxylation reactions of olefins are carried out in acetone with t-butanol (used to disolve and store the highly toxic OsO4) and tBuOOH to regenerate the OsO4 so alcohols are unaffected.
But t-butanol is a tertiary alcohol. Isn't that significantly different than primary or secondary alcohols?
Post by: Archer on December 05, 2013, 12:03:30 AM
But t-butanol is a tertiary alcohol. Isn't that significantly different than primary or secondary alcohols?
Good point! But the generated diol in the catalytic cycle remains intact so the only question is whether 1° alcohols are affected.
This article suggests that additives are needed to facilitate oxidation of 1° alcohols by OsO4
http://www.sciencedirect.com/science/article/pii/0021951780903528
Post by: zsinger on December 05, 2013, 12:18:10 AM
I am going to try the Swern tomorrow in my lab, and was wondering if you had any tips/why it is easier? Separations easier? Thanks.
-Z
Post by: Archer on December 05, 2013, 09:34:11 AM
I am going to try the Swern tomorrow in my lab, and was wondering if you had any tips/why it is easier? Separations easier? Thanks.
As with everything it can be substrate dependent, what are you oxidising?
You have to keep the temperature low (-60°C) but the reaction is clean and the reaction bi-products are either gaseous or pretty volatile.
Only issue with my protocol is that the excess of DMSO (2.2 molar excess) can be difficult to remove if your product is particularly volatile or water soluble.
I had to make this molecule
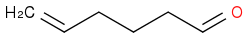
Which Swern did not work out for me and so I resorted to PCC in DCM.
Post by: discodermolide on December 05, 2013, 09:36:06 AM
You really should give TEMPO/bleach a go. I have a procedure if you want to try it.
Post by: Archer on December 05, 2013, 10:17:06 AM
@Archer:
You really should give TEMPO/bleach a go. I have a procedure if you want to try it.
Happy to try anything new, please let me know either on here or PM.
Thanks
Post by: AlphaScent on December 05, 2013, 03:35:10 PM
I would take that procedure if you are willing. I am being asked to scale up a fickle oxidation of (4Z,2E)-Octadien-1-ol.
PCC on 10-20 grams is ok. Still a pain in the ass. It is done at like 0.1 M so a lot of DCM is involved and the work-up is hell even on that 20 grams. 100 or more is out of the question.
I would really enjoy reading it!!
PM me it or I can give you my email.