Post by: bioengineer123 on March 11, 2015, 12:21:10 AM
ch3ch2ch2Och=o with brmgCh2ch(ch2)2 (isobutyl group with grignard reagent) and then brmg-ch3 in excess of water.
I think that the isobutyl will break the double bond of the oxygen and bond to the previously aldehyde carbon, and a water will drop off a hydrogen to become and alcohol. But im not sure what will happen in the second step, i thought that grignard reagents could react with alcohol but that doesnt seem to match up with the nmr data i was given for the product. any ideas here?
Post by: Hunter2 on March 11, 2015, 02:05:17 AM
Post by: sjb on March 11, 2015, 03:22:27 AM
so in the following reaction im having a few problems.
ch3ch2ch2Och=o with brmgCh2ch(ch2)2 (isobutyl group with grignard reagent) and then brmg-ch3 in excess of water.
I think that the isobutyl will break the double bond of the oxygen and bond to the previously aldehyde carbon, and a water will drop off a hydrogen to become and alcohol. But im not sure what will happen in the second step, i thought that grignard reagents could react with alcohol but that doesnt seem to match up with the nmr data i was given for the product. any ideas here?
If your first compound is
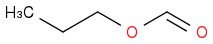 then this isn't an aldehyde, but an ester. With this in mind, does that help?
then this isn't an aldehyde, but an ester. With this in mind, does that help?