Post by: Consequentium on November 14, 2015, 04:29:57 PM
I'm attempting to identify an unknown substance using common spectroscopy techniques. All of the data is "real" in the sense that it wasn't collected from various sources and presented to me as a virtual problem. I personally conducted the IR, NMR, and melting point experiments. Only the GC-MS was done elsewehere and given to me. The substance is supposed to be pure. The GC didn't detect any impurities.
This problem has me tearing my hair out primarily because I cannot make sense of the mass spec data in light of the other data. Here's a quick overview of my work and my thinking so far:
IR:
Strong absorption at 1667cm is indicative of carbonyl, there are some weak absorptions that look like alehyde peaks just under 3000.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FmHfxTaF.jpg&hash=7bfb8940df18720accf5234c55381825269c2af8)
1H NMR:
Singlet ~10 tells me aledhyde is still likely. I doubt the most upfield singlet peak is aromatic, but it could be benzylic -CH2 attached to an electronegative atom. The others are aromatic and suggest 1,3,4 trisubstituted bezaldehyde.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FZyZ99at.jpg&hash=63fe0ea8efca3d1299d4b740b389f0e5dd076562)
Expansion:
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2F5E2xFlZ.jpg&hash=8ed85fb679b139c560c1dc10f661af005cd6961f)
13C NMR:
Pretty supplementary to 1H NMR: Gives me 8 unique carbons to play with and this goes well with my trisubstitution theory if there's a benzylic heteroatom attached to a carbon.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2Fg78mGhH.jpg&hash=62cbb4b3535101b18b419503d1b6428f91cb955f)
MS:
For everyone's sake, I won't swear at this thing as much as I'd like to. It's seriously the worst part of this. the NMRs and IR give me a tidy little theory which is utterly destroyed by this stupid thing. I can't massage the data here. It's fundamentally incompatible. A 149 M-peak is simply not enough to give me an 8 carbon benzaldehyde. The M+2 peak suggests chlorine due to the ratio. The aromatic signature in NMR is very distinct and can't be anything else. With a base peak of 149, subtracting for chlorine and oxygen, there is virtually no mass left to work with that doesn't leave me with either something too saturated to be aromatic, or something with a baffling remainder. Aldehydes sometimes have an M-1 peak that's stronger than the M-peak, but in EI MS, they're supposed to hold together fairly well. I briefly considered an alcohol, since they disintegrate in EI, but it would be shifted more downfield and the integration would be completely off. Also, no broad peak in IR. Since I can't make sense of the MS data, I made my best guess without it, but I really suspect I'm missing something here.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FCyLiHbo.jpg&hash=7a580a4a22fb8f16e0b5ea7abb93e2be2cbbcd30)
Finally the MP is ~35-40. Low, but it's solid at room temp. White, with big crystals that I had to grind down to fit in the NMR tube.
This is what I'm thinking so far, based on the reasoning:
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FR5Dkv5J.png&hash=7c4b60c305e3d4e4c249a778676de22f708017cb)
This fits well with NMR and IR, but it's the MS that has me scratching my head. It is my albatross, my White Whale, my Everest.
Any hints as to what I'm missing would be much appreciated.
Post by: AWK on November 14, 2015, 06:26:03 PM
two chlorine atoms M/(M+2)/(M+4)~ 9:6:1
In my opinion peak 151 is to high.
Post by: Consequentium on November 14, 2015, 11:10:11 PM
one chlorine atom M/(M+2)~ 3:1
two chlorine atoms M/(M+2)/(M+4)~ 9:6:1
In my opinion peak 151 is to high.
As in, you think it's the molecular ion? Throwing a Bromine or extra chlorine in there only seems to make the chemical formulas less possible. If 151 is the molecular ion, I can get a formula of C8H9NO2. This presents a different problem as one of the oxygens has to be in the aldehyde, and there can only be five sites of unsaturation. NH2, in my limited experience, shows up pretty broad in IR. Also a methoxy would integrate for three hydrogens. I didn't like it when I put a ring on it, (epoxide) and turning it into a heterocycle... saturation and integration were wrong.
I've literally been thinking about this problem all day. Maybe that's why I can't figure it out. It's driving me batty, and the worst part is I actually don't think it's that hard. Or at least it's not supposed to be.
Post by: Consequentium on November 15, 2015, 07:11:27 PM
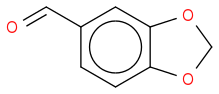
I'm pending a trip to the university library to look up data on Scifinder that will verify, but it makes perfect sense!
Thanks, AWK! All I needed was that gentle nudge and some time to think about it. 150 was the base peak all along, but the spectrum doesn't have the resolution to quite see that if the peaks aren't labelled. Fragmentation of an aldehyde group accounts for the 121 peak. I'll have to look into it more carefully before I'm sure, but I finally think I got it. The melting point makes sense as well.
Post by: AWK on November 16, 2015, 06:50:15 PM
Post by: Consequentium on November 17, 2015, 09:47:25 PM
http://www.massbank.jp/jsp/Dispatcher.jsp?type=disp&id=JP005884&site=10
Nice. I used SDBS already, but I'll bookmark that for future use.