Post by: INeedSerotonin on October 12, 2019, 07:16:58 PM
Hello
I have this exercise in which I have to say how many isomers are there in this molecule (if the aromatic ring cannot be removed). The answer is 4, but I'm unsure as to how can someone find this.
Could you guys give me any tips here?
Thanks
Post by: AWK on October 13, 2019, 01:39:05 AM
2 Move the methyl group to different positions of the phenyl ring.
I can see 5 isomers.
Post by: sjb on October 13, 2019, 05:26:47 AM
Post by: INeedSerotonin on October 13, 2019, 08:28:55 AM
1. Swap the methyl group with the amino group.
2 Move the methyl group to different positions of the phenyl ring.
I can see 5 isomers.
I can see only four isomers
1) The original molecule (CH3 - NH - benzene)
2) NH2 - ortho-methyl-benzene
3) NH2 - meta-methyl-benzene
4) NH2 - para-methyl-benzene
What is the fifth one? I cannot find it ??? ??? :(
Depending on how you define "the aromatic ring can not be removed" there are possibly more than 5. Not sure that would be covered at school level though.
What is the other possible interpretation? I'm still on High School level of chemistry.
Post by: AWK on October 13, 2019, 09:29:31 AM
o-, m-, p- toluidine, benzylamine
Post by: INeedSerotonin on October 13, 2019, 10:02:45 AM
H2NCH2-C6H5
o-, m-, p- toluidine, benzylamine
Thank you!
Could I say that there are 6 isomers in total, counting the original molecule too?
Post by: AWK on October 13, 2019, 10:19:41 AM
Post by: INeedSerotonin on October 13, 2019, 10:23:48 AM
No, together with N-methyl aniline you have 5 isomers of benzene serie.
Ok! Thanks! ;)
Post by: chenbeier on October 13, 2019, 11:03:18 AM
Post by: sjb on October 13, 2019, 11:04:59 AM
Depending on how you define "the aromatic ring can not be removed" there are possibly more than 5. Not sure that would be covered at school level though.
What is the other possible interpretation? I'm still on High School level of chemistry.
Consider compounds like
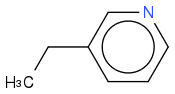 or even
or even 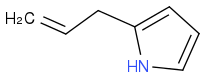
Post by: sjb on October 13, 2019, 11:05:33 AM
You can also move the nitrogen into the aromatic ring, you can have o-,m-, and p- Methylpyridine. Also called Picoline. So 8 Isomeres in total.
These would only have 6 carbons, but yes.
Post by: chenbeier on October 13, 2019, 11:10:36 AM
Post by: chenbeier on October 13, 2019, 11:12:21 AM
You can also move the nitrogen into the aromatic ring, you can have o-,m-, and p- Methylpyridine. Also called Picoline. So 8 Isomeres in total.
These would only have 6 carbons, but yes.
I changed it to the ethylproduct and add the dimethyl-isomeres above.