Post by: tunkor on March 24, 2020, 06:08:25 AM
I am kinda shooting in the dark here. I'm a mechanical engineering student with very little knowledge of chemistry. I'm trying to figure out a proces for casting a 3d printed model by directly pouring molten iron (1400C) on top of it.
The model is coated by a ceramic coating and put in a sand container. The molten metal 'interacts' with the PLA model by melting and evaporating(?) the PLA. The metal will take the shape of the 3D printed model inside the ceramic coating shell, leaving a casted product. I have done a few tests which came out decent, I am however eager to know about what exactly happens when these two materials (fluid metal, and PLA) interact with eachother.
What happens to the PLA, how fast does this happen, what kind of rest products are left behind, how much gas does the PLA produce? Information like this would be useful for me to know, to maybe screen for other printing materials other than PLA. I'm basically trying to analytically see what happens with the material.
For example, I want as little gas production from the material, because this gas needs to leave via the ceramic coating. So if I can screen a material beforehand this would be useful.
Would anyone know how I can figure this out, maybe some useful online tools/databases/literature?
I hope I am asking this in the right place!
Thanks in advance.
Kind regards.
Post by: Borek on March 24, 2020, 06:01:27 PM
The exact outcome is insanely difficult to predict, this is one of these cases where the experimental approach is the only viable way of getting into details.
Post by: Enthalpy on March 24, 2020, 06:47:26 PM
I can't tell if the same is done with plain polymer like PLA nor if it can succeed.
- The amount of PLA needs much heat to decompose, possibly more than hot iron brings.
- The decomposition of PLA would leave a huge amount of carbon. Probably, iron can't fill the mold, but if iron achieves to decompose the PLA, too much carbon in iron will spoil its properties.
What the reaction is: mainly a pyrolysis, which is a pedant word for "makes a bunch of unknown dirty things". The big heat breaks all big molecules, the atoms rearrange in small molecules and often in graphite. From the C3H4O2 period, 2×CO may escape and possibly carry some hydrogen away. From the remaining C, some may leave as small hydrocarbons, but I suppose some remains as graphite.
By the way, pyrolysis products are always a bit toxic. Nothing tragic, but better do it outside, or in a well ventilated area, without making a chicha of it. If this shall become a regular activity, you need some protective measures.
Post by: Borek on March 24, 2020, 08:17:12 PM
It that process already used?
I have done a few tests which came out decent
Post by: tunkor on March 25, 2020, 06:18:45 AM
This process uses a foam traditionally, polystyrene foam. It works because the same volume holds little foam mass or much iron mass. Polystyrene leaves uncontrolled dirty things (mostly carbon) whose small proportion in iron is tolerable.
I can't tell if the same is done with plain polymer like PLA nor if it can succeed.It that process already used? What lets you suppose that the dirty things will escape through the ceramic?
- The amount of PLA needs much heat to decompose, possibly more than hot iron brings.
- The decomposition of PLA would leave a huge amount of carbon. Probably, iron can't fill the mold, but if iron achieves to decompose the PLA, too much carbon in iron will spoil its properties.
What the reaction is: mainly a pyrolysis, which is a pedant word for "makes a bunch of unknown dirty things". The big heat breaks all big molecules, the atoms rearrange in small molecules and often in graphite. From the C3H4O2 period, 2×CO may escape and possibly carry some hydrogen away. From the remaining C, some may leave as small hydrocarbons, but I suppose some remains as graphite.
By the way, pyrolysis products are always a bit toxic. Nothing tragic, but better do it outside, or in a well ventilated area, without making a chicha of it. If this shall become a regular activity, you need some protective measures.
The lost foam proces is what I'm 'copying' from. I am using a PLA which has a foaming additive to it, achieving very low weight printed parts. This was kinda the basis for starting this experiment, because the total density of the part can come pretty close to what a foam model would be. The difference being that the PLA model is mostly hollow, whereas the foam is homogeneous. This brings a difference in how the metal will fill the void (like the metal flowing too fast), but that's another story I am trying to experiment with.
How much energy does it take te decompose PLA compared to PS? Is this what activation energy means? How much carbon does PLA leave compared to PS?
I tried a few tests, but haven't checked the casted part for any properties (but this is in the works). The parts were pretty small and simple (a rounded off cube) but mostly casted fine, some parts did not fill up nicely. But I will be testing new PLA models soon which hopefully have even less mass which has to be burned.
By the way thank you so much for taking the time to reply, I really appreciate it!
Post by: tunkor on March 25, 2020, 06:21:45 AM
By the look of it I would expect decomposition to a mixture of gases (CO, CO2, ethylene, water, some of them at these temperatures would immediately burn on contact with air), and perhaps some elemental carbon (which can easily dissolve in the iron). Assuming simple decomposition to CO2 and ethylene simple stoichiometry is all you need to calculate amounts.
The exact outcome is insanely difficult to predict, this is one of these cases where the experimental approach is the only viable way of getting into details.
Thank you for your reply!
So if you simplify the reaction, the PLA would decompose into CO2 and ethylene?
Post by: Borek on March 25, 2020, 09:04:14 AM
So if you simplify the reaction, the PLA would decompose into CO2 and ethylene?
Yes, at first stage. Just remember - this is just an educated guess with all possible disclaimers, experiment can easily prove it wrong. Search for a thermogravimetric curve for PLA, it should give some insight into what is happening.
How much energy does it take te decompose PLA compared to PS?
You can try to use Hess law to estimate the numbers.
Quote
Is this what activation energy means?
No, activation energy is related to the reaction speed, you are looking for reaction enthalpy.
Quote
How much carbon does PLA leave compared to PS?
No way to tell - thermal decomposition (pyrolysis) is a process consisting of many quite random reactions, with lots of side products.
I tried a few tests, but haven't checked the casted part for any properties (but this is in the works). The parts were pretty small and simple (a rounded off cube) but mostly casted fine, some parts did not fill up nicely. But I will be testing new PLA models soon which hopefully have even less mass which has to be burned.
By the way thank you so much for taking the time to reply, I really appreciate it!
[/quote]
Post by: Enthalpy on March 25, 2020, 09:20:22 AM
Within a ceramic mould deposited on the foam, I expect very little air mass is available, so the compounds present are together very reductive. I expect CO rather than CO2, which will pick some hydrogen to form things like H2CO. The rest of PLA [-C3H4O2-]n would be four H for one C if no hydrogen combined with CO.
If the foam has closed cells, the foaming gas contributes to the gross composition. With open cells, it will be air. The amount isn't huge, but it can increase radically the toxicity of the evolved gas. If polystyrene is foamed by a halogenated gas, this makes toxic fumes.
What do the up to four H for one C do? I won't risk a prediction. There will be some graphite and high-carbon compounds, but the amount doesn't result from a simple reaction equation. The rest can evolve as CH4, C2H4 and thousands more.
==========
Out of curiosity, I've let Propep compute the equilibrium products of hot polymers brought to 1400°C and 1atm. This is NOT what happens in your case, because the casting process is very far from an equilibrium. Also, iron will catch some oxygen. The evolved gases recombine as they cool down, for instance CH3, H, HCO disappear for sure. These are molar fractions. Propep hides the least abundant species. I've take polybutadiene for its small H/C ratio. Even smaller at polystyrene, but Propep failed.
----- POM
7.2750e-007 CH3
2.2516e-004 CH4
4.9990e-001 CO
5.9635e-005 CO2
1.4937e-005 C2H2,acetylene
1.4589e-008 CH2CO,ketene
4.6162e-007 C2H4
8.0184e-005 H
3.4620e-008 HCO
4.9952e-001 H2
1.0892e-007 HCHO,formaldehy
1.9706e-004 H2O
----- PLA
8.0500e-007 CH3
2.4911e-004 CH4
4.0002e-001 CO
3.4496e-005 CO2
2.2871e-005 C2H2,acetylene
1.6148e-008 CH2CO,ketene
7.0665e-007 C2H4
6.4143e-005 H
2.7699e-008 HCO
3.9954e-001 H2
8.7137e-008 HCHO,formaldehy
1.1394e-004 H2O
Condensed species
1.9995e-001 C(gr)
----- Polybutadiene
1.2192e-006 CH3
5.3350e-004 CH4
2.4496e-005 C2H2,acetylene
1.5134e-006 C2H4
4.8583e-005 H
4.2792e-001 H2
Condensed species
5.7147e-001 C(gr)
----- PS
Propep failed to find an equilibrium.
Post by: tunkor on March 25, 2020, 12:10:30 PM
So if you simplify the reaction, the PLA would decompose into CO2 and ethylene?
Yes, at first stage. Just remember - this is just an educated guess with all possible disclaimers, experiment can easily prove it wrong. Search for a thermogravimetric curve for PLA, it should give some insight into what is happening.How much energy does it take te decompose PLA compared to PS?
You can try to use Hess law to estimate the numbers.QuoteIs this what activation energy means?
No, activation energy is related to the reaction speed, you are looking for reaction enthalpy.QuoteHow much carbon does PLA leave compared to PS?
No way to tell - thermal decomposition (pyrolysis) is a process consisting of many quite random reactions, with lots of side products.
Thanks, can you elaborate on the law of Hess? The last chemistry I had was in high school 7 years ago, so I apologize for being so ignorant.
Post by: Borek on March 25, 2020, 02:27:56 PM
Thanks, can you elaborate on the law of Hess? The last chemistry I had was in high school 7 years ago, so I apologize for being so ignorant.
Any general chemistry 101 textbook should have an introduction, this is a relatively basic concept (which I am saying not to stress your lack of knowledge but to signal that you should be able to grasp the idea even without having a solid chemistry background :) ).
You may have problems finding enthalpy of formation for PLA, it can be estimated from bond energies though. Enthalpy of formation of a compound is equal to sum of bond energies, these can be found tabulated. Far from perfect, better than nothing.
Post by: Enthalpy on March 26, 2020, 07:12:03 AM
Post by: tunkor on March 26, 2020, 07:20:39 AM
I doubted that the heat brought by molten iron would suffice to destroy plain polymer. But with polymer foam, I could easily drop numerical checking.
It's filament with a foaming agent, at a certain temperature the solid PLA filament will foam a little bit.
(https://i.imgur.com/8OuwJg0.png)
In that picture on the left is a single wall of normal PLA, and on the right is a single wall of foaming PLA. Volume can increase by 3x using this material according to the manufacturer, by increasing temperature and decreasing material flow lightweight parts are possible. So this would mean less mass to burn.
Post by: Enthalpy on March 27, 2020, 12:23:10 PM
Liquid ethyl acetate has ΔHf=-479kJ/mol. One C-C bond more and H2 less add 44kJ/mol, so PLA has ΔHf=-435kJ/mol of -C3H4O2-.
If we were to believe Propep, it decomposes to 1*C + 2*CO + 2*H2 whose ΔHf=-221kJ/mol. The heat of the rest is negligible.
So the pyrolysis of 1 mol of -C3H4O2-, or 72g, absorbs 214kJ/mol. That's 3MJ/kg. If the foam is 440kg/m3 dense, it absorbs 1.3GJ/m3.
The heat capacity of molten Fe is around 45J/mol/K = 800J/kg/K = 5.6MJ/m3/K. Iron being supposedly less than 230K above its melting point, some iron will solidify.
The heat of fusion of Fe is roughly 13kJ/mol = 230kJ/kg = 1.6GJ/m3. This suggests that most iron solidifies by decomposing PLA. Bad.
A much lighter foam would be welcome.
Post by: tunkor on March 27, 2020, 03:44:31 PM
So PLA is still rather dense. My estimation of the decomposition heat follows.
Liquid ethyl acetate has ΔHf=-479kJ/mol. One C-C bond more and H2 less add 44kJ/mol, so PLA has ΔHf=-435kJ/mol of -C3H4O2-.
If we were to believe Propep, it decomposes to 1*C + 2*CO + 2*H2 whose ΔHf=-221kJ/mol. The heat of the rest is negligible.
So the pyrolysis of 1 mol of -C3H4O2-, or 72g, absorbs 214kJ/mol. That's 3MJ/kg. If the foam is 440kg/m3 dense, it absorbs 1.3GJ/m3.
The heat capacity of molten Fe is around 45J/mol/K = 800J/kg/K = 5.6MJ/m3/K. Iron being supposedly less than 230K above its melting point, some iron will solidify.
The heat of fusion of Fe is roughly 13kJ/mol = 230kJ/kg = 1.6GJ/m3. This suggests that most iron solidifies by decomposing PLA. Bad.
A much lighter foam would be welcome.
Guys thanks so much to both of you! This has given me a lot more insight already :)
Post by: Enthalpy on April 01, 2020, 07:18:36 AM
And: double-checking my computation wouldn't hurt.
Post by: tunkor on April 01, 2020, 07:39:19 AM
Please don't consider my estimate as a prediction! Propep computes equilibria and your process is very far from equilibrium. So if you observe a too fast solidification, you have a qualitative explanation, nothing more. Enough to seek a lighter foam. If less carbon in iron is desired, POM-H foam could be interesting.
And: double-checking my computation wouldn't hurt.
Currently I am trying to apply the decomposition of PLA to simple models EPS decomposing. Then I hope to verify these predictions in real life scenarios.
What I need for these models is the energy required for decomposing PLA. From what I gathered in an earlier reply you calculated the enthalpy of formation (an indicator for the energy required for decomposition?) by looking at the enthalpy of formation of ethyl acetate and compare the energy bonds with PLA.
I don't understand how you calculated this exaclty however, if I compared PLA with ethyl acetate I see that PLA has a C = C bond, one C less and 2 H2 less. Could you clarify this for me? It would help me a lot!
And the other thing I have to find out is the amount of gas generated, to see what the pressure will be in the gap between the PLA and the metal. But I am still looking for info about that.
Post by: Enthalpy on April 02, 2020, 09:30:43 AM
You're absolutely right that ethyl acetate has one C too much. I botched that. "Double check" was more than rhetoric here: it's the base of science.
Methyl acetate would be the right starting material
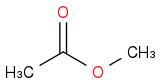
to compare with the PLA period, gratefully pinched from Wiki and appended here. Methyl acetate resembles the PLA period: same ester group, as many carbons and bonds, just two C-H becoming a C-C and an H-H, so few changes of small energy suffice.
From tables, the heat of formation of methyl acetate is ΔH=-446kJ/mol. For the liquid, not the solid, slight inaccuracy. With ethyl acetate, my error was 33kJ/mol, over the previous 214kJ/mol heat of decomposition.
The formal transformation to PLA period is the same as from two octanes becoming hexadecane and hydrogen. 2* (-250kJ/mol) becomes -456kJ/mol and 0kJ/mol, this absorbs 44kJ/mol. We could skimp on primary and secondary carbons, but I won't since this makes 5kJ/mol difference. Here we get a heat of formation ΔH=-402kJ/mol for the PLA period.
Again from tables, CO has ΔH=-110.5kJ/mol while H2 and solid C have zero by convention. So the decomposition to 1*C + 2*CO + 2*H2 absorbs 402-2*110.5=181kJ/mol, not 214.
However, this is at 298K, but here the reaction products exit at about 1500°C - I shouldn't have botched that one. Misusing at heat the capacity at room temperature (OK for H2 and CO, not for CO2 or H2O)
https://en.wikipedia.org/wiki/Table_of_specific_heat_capacities
8,5 + 2×29,1 + 2×28,8 = 124J/mol/K for 1*C + 2*CO + 2*H2
heating from 0 to 1500°C absorbs further 186kJ/mol.
So from one PLA period at room temperature to 1*C + 2*CO + 2*H2 at 1500°C, it takes about 367kJ/mol of C3H4O2 weighing 72g, or 5MJ/kg.
==========
The amount of gas is easy. 2*CO + 2*H2 at 1500°C, you can apply the ideal gas law.
The pressure is about impossible to evaluate. Your mould needs vents or it will explode, and the foam must be weak enough to open passages. The pressure results from very dynamic processes, not from an equilibrium.
Post by: tunkor on April 02, 2020, 04:33:24 PM
I compare the heat of formation of the "reactants" (PLA) and the "products" (CO, H2, C) to obtain the heat of reaction (here a pyrolysis or decomposition). It's a difference, not forgetting numbers of moles. Sign conventions exist, the useful part is that the present decomposition absorbs heat.
You're absolutely right that ethyl acetate has one C too much. I botched that. "Double check" was more than rhetoric here: it's the base of science.
Methyl acetate would be the right starting material
to compare with the PLA period, gratefully pinched from Wiki and appended here. Methyl acetate resembles the PLA period: same ester group, as many carbons and bonds, just two C-H becoming a C-C and an H-H, so few changes of small energy suffice.
From tables, the heat of formation of methyl acetate is ΔH=-446kJ/mol. For the liquid, not the solid, slight inaccuracy. With ethyl acetate, my error was 33kJ/mol, over the previous 214kJ/mol heat of decomposition.
The formal transformation to PLA period is the same as from two octanes becoming hexadecane and hydrogen. 2* (-250kJ/mol) becomes -456kJ/mol and 0kJ/mol, this absorbs 44kJ/mol. We could skimp on primary and secondary carbons, but I won't since this makes 5kJ/mol difference. Here we get a heat of formation ΔH=-402kJ/mol for the PLA period.
Again from tables, CO has ΔH=-110.5kJ/mol while H2 and solid C have zero by convention. So the decomposition to 1*C + 2*CO + 2*H2 absorbs 402-2*110.5=181kJ/mol, not 214.
However, this is at 298K, but here the reaction products exit at about 1500°C - I shouldn't have botched that one. Misusing at heat the capacity at room temperature (OK for H2 and CO, not for CO2 or H2O)
https://en.wikipedia.org/wiki/Table_of_specific_heat_capacities
8,5 + 2×29,1 + 2×28,8 = 124J/mol/K for 1*C + 2*CO + 2*H2
heating from 0 to 1500°C absorbs further 186kJ/mol.
So from one PLA period at room temperature to 1*C + 2*CO + 2*H2 at 1500°C, it takes about 367kJ/mol of C3H4O2 weighing 72g, or 5MJ/kg.
==========
The amount of gas is easy. 2*CO + 2*H2 at 1500°C, you can apply the ideal gas law.
The pressure is about impossible to evaluate. Your mould needs vents or it will explode, and the foam must be weak enough to open passages. The pressure results from very dynamic processes, not from an equilibrium.
Thank you so much! I really appreciate you taking the time to help me with this.
I have one another question, I've been looking at articles about the decomposition of PLA. I see a lot about different types of hydrocarbons being emissions of PLA decomposition, but these are from TGA done at 400C for example. You mention the products are CO, H2 and C. Is this what the products of decomposition are after reaching 1500C? Any partical reason the products decompose to those ones?
Thanks again for the tremendous *delete me*
Post by: Enthalpy on April 03, 2020, 09:22:15 AM
1500°C or 1600°C suffice to break most bonds, especially C-C and C-H. No organic material operates at this temperature, only ceramics and metals. This lets the atoms rearrange in species (not even molecules) according to the thermodynamic equilibrium. Propep, a combustion estimation software for rocket engines, computes exactly that: it neglects how the atoms were combined prior to heat, and seeks a mathematical equilibrium among the hundreds of species known from tables and capable to form from the available atoms.
If PLA were brought uniformly to 1500°C, I'm confident the decomposition products would match Propep's estimation. The difference is that your process is very dynamic: some PLA not in direct contact with iron decomposes well before reaching the final temperature, and the decomposition products escape by deforming the surrounding material. These products have a different composition.
Post by: billnotgatez on April 06, 2020, 04:09:22 AM
Post by: tunkor on April 15, 2020, 09:20:08 AM
The decomposition products depends fundamentally on the temperature, and also on the pressure. At not too high temperature, like 400°C, they depend also on the heat history, because the detailed processes still matter: heat doesn't suffice to break and rearrange any compound, instead, the reactions with the least hurdles proceed more easily.
1500°C or 1600°C suffice to break most bonds, especially C-C and C-H. No organic material operates at this temperature, only ceramics and metals. This lets the atoms rearrange in species (not even molecules) according to the thermodynamic equilibrium. Propep, a combustion estimation software for rocket engines, computes exactly that: it neglects how the atoms were combined prior to heat, and seeks a mathematical equilibrium among the hundreds of species known from tables and capable to form from the available atoms.
If PLA were brought uniformly to 1500°C, I'm confident the decomposition products would match Propep's estimation. The difference is that your process is very dynamic: some PLA not in direct contact with iron decomposes well before reaching the final temperature, and the decomposition products escape by deforming the surrounding material. These products have a different composition.
That makes sense, man there's a lot to this! How can I compute the reaction products myself in Propep? I see there's no option for PLA in the list.
Post by: tunkor on April 15, 2020, 09:23:09 AM
I have been following this thread and wonder if you considered using the standard lost wax procedure of heating the investment first before pouring in the final metal. We do it at 1000 F for overnight. The pour hole and sprues allow the wax to flow out and/or burn out (investments are holes down). We vacuum out the pour hole and sprues to remove any residual burn products and pour while the investment is somewhere below 500 F. Due to the lock down I am unable to reach out to my fellow casters to get their thoughts (which I would have added here).
Good question, investment casting with melting out the investment has some downsides from what I understand. With the investment still being inside the coating it will add to the total strength. So you can use a thinner coating, which also means a shorter lead time before you can start casting. I think lost foam casting also allows for larger casting products due to this reason.
Post by: Enthalpy on April 16, 2020, 07:07:54 AM
To compute an equilibrium, I check or fill in CPropepShell
Fixed pressure and temperature
Fixed chamber pressure
Fixed chamber temperature
and I give twice PLA as an ingredient, 500g+500g, because CPropepShell doesn't understand monopropellants.
I added PLA with a text editor anywhere in the propellants list propellant.dat as:
8043 Polylactic acid (zero Hf!) 3C 4H 2O 0 0 0 0 .0000]
The density .0000 in Lb/in3 (avoirdupois) is unnecessary for most purposes, and I kept 0 cal/g heat of formation in but-last column because it serves to compute the chamber temperature, but here we impose it.
The number of spaces matters in the line format and this forum may suppress some. Check that the new line aligns with the others. The line number 8043 plays no role known to me. C H O can be other elements and in a different sequence, apparently the usual chemical symbols.
POM exists already in the list as Delrin.
----------
Removing the foam before casting is a different process. Both serve. Removal would be difficult for some shapes and it takes more steps. Only some alloys like cast iron accept the carbon pollution. POM could be an answer to that, if a foam light enough is or can be made.