Post by: unofku on April 26, 2020, 02:02:16 PM
I think it might be
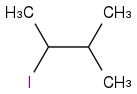 or
or 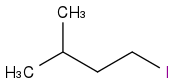 but I am really unsure.
but I am really unsure. If there is a kind person who can read the H-NMR and explain the signals to me ?
Post by: AWK on April 26, 2020, 02:11:57 PM
Post by: wildfyr on April 27, 2020, 09:18:58 AM
Post by: unofku on April 27, 2020, 09:22:30 AM
Post by: Babcock_Hall on April 27, 2020, 09:35:31 AM
Post by: unofku on April 27, 2020, 11:36:16 AM
H-NMR is a big struggle to me. I have worked out that the chemical shift will tell me which groups are present (eg. -CH3, aldehydes etc), and integration can tell me the ratio of the hydrogens and splitting informs of the neighboring hydrogens. I am struggling to relate all the concepts and really relate it to specific structures.
Post by: AWK on April 27, 2020, 12:23:40 PM
Post by: unofku on April 27, 2020, 12:41:30 PM
Would this then be the correct answer ?
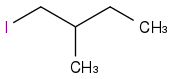
I also have a question about the part of the spectrum that doesn't show the number of peaks (at approx 1.35-1.38), how do I interpret this ?
Post by: AWK on April 27, 2020, 12:44:39 PM
Post by: wildfyr on April 27, 2020, 01:08:14 PM
I think you are making this much too complicated for yourself. I am no NMR genius but I could see what molecule it was at a glance once you IDed it as a mono iodoalkane. I think the peak at 1.3-1.4 is really tripping you up. How about I just whisper to you that its two pretty simple overlapping peaks that integrate to 4 total protons.
Post by: Babcock_Hall on April 27, 2020, 01:18:56 PM
Thank you for your reply!Chemical shift tells you about the environment of the hydrogen atoms. Integration tells you the number of hydrogens which are equivalent in chemical shift. But the number of separate peaks (ignoring fine structure) is just as important as the integral of each peak. It tells us the number of sets of equivalent nuclei. My interpretation of AWK's comments is that this is the direction that AWK's is leading you. Splitting tells you about the number of vicinal hydrogen atoms. Your answers were basically fine but needed a little sharpening up.
H-NMR is a big struggle to me. I have worked out that the chemical shift will tell me which groups are present (eg. -CH3, aldehydes etc), and integration can tell me the ratio of the hydrogens and splitting informs of the neighboring hydrogens. I am struggling to relate all the concepts and really relate it to specific structures.
Sometimes there are cases which don't exactly follow the splitting rules taught in introductory courses. That is the region around 1.3-1.4 ppm. I think that you can solve the problem without worrying about the reason for this appearance.