Post by: hairygorillaz on May 09, 2020, 06:02:21 AM
I have a question about 1-bromoethanol and whether it will undergo the iodoform reaction. I have two possible reaction pathways. (1) Oxidation to the acyl bromide, followed by hydrolysis and deprotonation to form an acetate anion. Or (2) Nucleophilic substitution of the bromide, forming a geminal diol, forming acetaldehyde upon losing water which then undergoes the iodoform reaction.
I'm more inclined for (1). Any thoughts? Thanks!
Post by: chenbeier on May 09, 2020, 06:13:40 AM
Post by: sjb on May 09, 2020, 08:32:02 AM
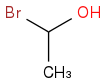 is quite unstable and will probably decompose to HBr and acetaldehyde / ethanal fairly quickly and probably won't get a chance to undergo the steps you've outlined
is quite unstable and will probably decompose to HBr and acetaldehyde / ethanal fairly quickly and probably won't get a chance to undergo the steps you've outlined
Post by: chenbeier on May 09, 2020, 09:47:36 AM
Post by: rolnor on May 09, 2020, 10:46:32 AM
https://en.wikipedia.org/wiki/Haloform_reaction