Post by: lukas.stib on July 04, 2020, 07:04:20 AM
I synthesized pentan-2-one from ethyl acetoacetate and sodium hydroxide. I did everything according to the instructions, where they used 10 g NaOH, 100 ml H2O and 15 g ethyl acetoacetate. For more product, I used three times everything, so 30 g NaOH, 300 ml H2O and 45 g ethyl acetoacetate. I refluxed for 2 hours. It is written in the instructions that 2 layers are to be formed after 2 hours refluxing, the oily layer is pentan-2-one. Unfortunately, nothing was created; I cooled the flask in cold water, and now, after 45 minutes, nothing still formed. Can you advise me what to do?
Thank you, Lukáš S.
Post by: sjb on July 04, 2020, 07:40:01 AM
Post by: lukas.stib on July 04, 2020, 08:22:18 AM
Post by: Babcock_Hall on July 04, 2020, 11:00:12 AM
Post by: lukas.stib on July 04, 2020, 11:35:59 AM
Post by: Babcock_Hall on July 04, 2020, 01:08:44 PM
Post by: rolnor on July 04, 2020, 01:47:46 PM
Maybe run the reaction in the same scale as in the reference to start with?
Post by: Babcock_Hall on July 04, 2020, 03:27:06 PM
Post by: lukas.stib on July 04, 2020, 04:07:23 PM
Post by: lukas.stib on July 05, 2020, 06:06:10 AM
I'm re-synthesizing pentan-2-one, which I started yesterday. Now I did not use 3 times the raw materials, but I used the amount in the instructions, so 10 g NaOH, 100 ml H2O and 15 g ethyl acetoacetate. Now I perform reflux for more than 2 hours, as they write in the instructions. Reflux is more than 3 hours, and still 2 layers have not formed. I let the flask cool to normal temperature, and there are still no 2 layers.
I don't know what I did wrong; on your recommendation, I left the reflux for more than 2 hours, and it still doesn't bleed the product. Do you know what I'm doing wrong?
Thank you, Lukáš S.
Post by: rolnor on July 05, 2020, 08:45:32 AM
Post by: lukas.stib on July 05, 2020, 09:39:42 AM
And also my thermometer, which is from China, is probably not accurate, so the exact temperature of the start of distillation may have been different.
Post by: AWK on July 05, 2020, 11:20:32 AM
Post by: lukas.stib on July 05, 2020, 01:05:06 PM
Post by: AWK on July 05, 2020, 01:20:34 PM
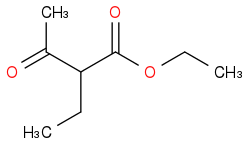
Post by: Babcock_Hall on July 05, 2020, 01:45:11 PM
Post by: lukas.stib on July 05, 2020, 01:53:39 PM
And i used this ester:
https://en.wikipedia.org/wiki/Ethyl_acetoacetate
Post by: AWK on July 05, 2020, 01:58:31 PM
Post by: lukas.stib on July 05, 2020, 02:01:54 PM
Post by: lukas.stib on July 05, 2020, 02:05:46 PM
Post by: AWK on July 05, 2020, 02:09:43 PM
Post by: lukas.stib on July 05, 2020, 02:31:14 PM
Post by: AWK on July 05, 2020, 02:40:47 PM
Post by: lukas.stib on July 05, 2020, 02:53:35 PM
So therefore explains why the boiling point in my distillation began at 61°C, and was felt after acetone; it was acetone !!!
OK, for pentan-2-one I must have ethyl-2-ethyl-3-oxobutanoate, or by oxidation of 2-pentanol by dichromate.
Post by: rolnor on July 05, 2020, 04:27:10 PM
Post by: lukas.stib on July 05, 2020, 04:45:32 PM
I can buy pentan-2-on, but I do it for fun, and when I buy it, I don't like it anymore...
Post by: AWK on July 06, 2020, 12:25:41 AM
Post by: lukas.stib on July 06, 2020, 02:36:21 AM
Post by: AWK on July 06, 2020, 02:56:02 AM
Post by: lukas.stib on July 06, 2020, 04:34:20 AM
Lukáš S.
Post by: billnotgatez on July 06, 2020, 02:40:49 PM
Post by: Babcock_Hall on July 06, 2020, 05:03:52 PM