Post by: Gameofketones on August 02, 2020, 02:11:50 AM
Post by: AWK on August 02, 2020, 04:39:01 AM
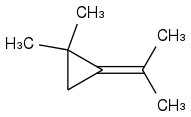
Post by: Enthalpy on August 03, 2020, 04:59:50 AM
how reasonable is a prediction for such unstable compounds?
Post by: AWK on August 03, 2020, 05:38:58 AM
For my personal information:There are many more unstable compounds that chemists work with. The derivative of the compound drawn by me (ring substituted propionate ester) is the pheromone of American cockroaches.
how reasonable is a prediction for such unstable compounds?
Post by: hollytara on August 04, 2020, 05:02:59 PM
Shapiro uses 2 equivalents of BuLi while Bamford-Stevens uses "base" in general, so can be done in protic or aprotic solvents. Both go through a Diazo intermediate, depending on conditions this may make a carbene (aprotic) or a carbocation (protic).
So imagine a carbene or carbocation was formed where the N attaches to the ring. How would that lead to what you find?
Post by: wildfyr on August 05, 2020, 10:12:15 AM
Post by: AWK on August 05, 2020, 12:05:34 PM
Totally unrelated, but how is =N-NH-Ts formed?Very easy. Ketone and tosylhydrazine in methanol, a few drops of conc. HCl, RT overnight.
Post by: wildfyr on August 05, 2020, 07:51:49 PM
Post by: Enthalpy on August 06, 2020, 03:22:07 PM
Sure. I saw tentative rocket fuels with cyclopropenes and spirohex-1-eneFor my personal information: how reasonable is a prediction for such unstable compounds?There are many more unstable compounds that chemists work with [...]
https://apps.dtic.mil/dtic/tr/fulltext/u2/a248465.pdf
But the authors easily recognized that they obtained 10 times the wrong compound and 1 time or 0 time the desired one, due to unpredictable rearrangements. That's why I wondered about predicting products.
Post by: AWK on August 06, 2020, 06:29:23 PM
Sure. I saw tentative rocket fuels with cyclopropenes and spirohex-1-eneFor my personal information: how reasonable is a prediction for such unstable compounds?There are many more unstable compounds that chemists work with [...]
https://apps.dtic.mil/dtic/tr/fulltext/u2/a248465.pdf
But the authors easily recognized that they obtained 10 times the wrong compound and 1 time or 0 time the desired one, due to unpredictable rearrangements. That's why I wondered about predicting products.
In this particular case, the pyrolysis of tosyhydrazone sodium as a synthesis method rather excludes the most strained endocyclic cyclopropenes or cyclobutenes. The endocyclic double bond (isopropylidene or isopropenyl) is a much better explanation for thermal rearrangement. Therefore, I immediately indicated 1,1-dimethyl-2-isopropylidenecyclopropane.
Post by: AWK on August 07, 2020, 02:24:59 PM
Quote
The endocyclic double bondIt should be: The exocyclic double bond
Post by: rolnor on August 07, 2020, 04:59:03 PM
Post by: AWK on August 07, 2020, 05:59:03 PM
This is not the correct answer.
Hollytara gave the correct name of this reaction.
Gameofketones should show his work. I showed the most probable structure as a result of carbocation rearrangement (base - MeONa in methanol).
Post by: Gameofketones on August 10, 2020, 04:30:45 AM
Post by: AWK on August 10, 2020, 05:36:57 AM