Post by: iwtdlol2020 on November 25, 2020, 02:49:45 AM
There is an unknown compound. It was large with different functional groups. You divided the molecules into significant parts by reacting it with different reagents. Using analytical instruments, the smaller parts were identified: methyl, a hydrocarbon chain of 10 carbons with two double bonds and a triple bond, ethyl, propyl
The methyl, ethyl and propyl groups are found to have 2:2:1 mass ratio respectively per molecule of the longest hydrocarbon.
You are tasked to suggest 2 possible structures of the unknown compound by combining all the groups mentioned above.
If the longest hydrocarbon is changed to benzene, explain the changes in the name and properties that will happen.
So my question is:
What does it mean by there is methyl, ethyl, and propyl groups with a ratio of 2:2:1 "PER MOLECULE" of the longest hydrocarbon? Does it mean the groups are to be attached to a Carbon molecule?
Post by: sjb on November 25, 2020, 04:13:20 AM
Post by: Borek on November 25, 2020, 04:25:58 AM
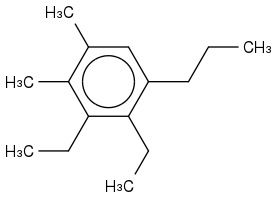
methyl:ethyl:propyl:main chain = 2:2:1:1
Post by: iwtdlol2020 on November 25, 2020, 05:57:26 AM