Post by: Win,odd Dhamnekar on February 03, 2021, 08:14:31 AM
[tex] H_2 + CO +RCH = CH_2 \rightarrow RCH_2CH_2CHO \tag{1}[/tex]
[tex] H_2 + RCH_2CH_2CHO \rightarrow RCH_2CH_2CH_2OH \tag{2}[/tex]
What is R in the reaction(1)?
The product doesn't seem to look alcohol
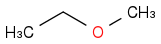 in reaction (2). What is R in reaction (2)?
in reaction (2). What is R in reaction (2)?
Post by: AWK on February 03, 2021, 08:59:47 AM
Depending on the reaction conditions, the product may be formate esters (not methyl ethers).
Post by: Orcio_87 on March 06, 2021, 02:58:08 PM