Post by: ComradeLenin on November 24, 2021, 01:36:29 PM
Post by: Babcock_Hall on November 24, 2021, 02:13:32 PM
Post by: Orcio_87 on November 24, 2021, 02:41:23 PM
Quote
Can anyone explain me why phenol's melting point is lower than benzene's one? Many thanks in advanceBecause it is higher.
Post by: billnotgatez on November 24, 2021, 10:32:15 PM
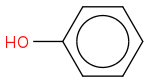
Properties
Chemical formula C6H6O
Molar mass 94.113 g/mol
Density 1.07 g/cm3
Melting point 40.5 °C (104.9 °F; 313.6 K)
Boiling point1 81.7 °C (359.1 °F; 454.8 K)
Benzene
Properties
Chemical formula C6H6
Molar mass 78.114 g·mol−1
Density 0.8765(20) g/cm3[2]
Melting point 5.53 °C (41.95 °F; 278.68 K)
Boiling point 80.1 °C (176.2 °F; 353.2 K)
From WIKI