Post by: Enthalpy on August 13, 2011, 02:10:46 PM
Boctane is a synthetic rocket fuel designed to improve performance over kerosene.
Finally, I could find it to be bi-cyclobutane C4H7-C4H7, Smiles C1CCC1C2CCC2 (it's not bicyclo[3.3.0]octane aka octahydropentalene)
http://www.freepatentsonline.com/6415596.html
and hand-estimate its heat of formation to +35.4kJ/mol, not far from +55.1kJ/mol claimed there
http://www.propulsion-analysis.com/downloads/thermo/thermo.inp
At +35.4kJ/mol it would bring 4s more specific impulse than kerosene Rp-1 and 7s more than Jp10, combined with a higher density, and might be used by existing rocket stages, fine. As good as Syntin, probably cheaper.
First, would you know more: density, melting point, boiling point, flash point...?
And: how safe and cheap could it be to produce in hundreds of tons?
I believe cyclobutane is a by-product of natural gas or oil, isn't it? So could maybe a chlorine substitute a hydrogen, and later a chlorocyclobutane fuse with a cyclobutane? Is that safe and cheap, like 1M$ for 100t? Better ideas?
By the way, I'm not studying chemistry, so ready-to-use answers would be just fine. Thanks!
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on August 15, 2011, 03:42:19 PM
Can you imagine a way to transform cyclobutane into cyclobutyl-cyclobutane? Substitute first with a bromine, maybe? Or an alcohol? Something workable in the 100's of tons.
Post by: Honclbrif on August 15, 2011, 04:32:23 PM
I'll be right back.
Post by: orgopete on August 15, 2011, 07:09:40 PM
By the way, I'm not studying chemistry, so ready-to-use answers would be just fine.
…
Can you imagine a way to transform cyclobutane into cyclobutyl-cyclobutane? Substitute first with a bromine, maybe? Or an alcohol? Something workable in the 100's of tons.
How would you make ethane from methane in the 100's of tons? I won't even ask for it to be cheap, yet.
Wouldn't dicyclobutylacetylene be better yet?
Post by: Enthalpy on August 15, 2011, 10:05:55 PM
Yes, just hold on while I tell you how to make millions of dollars worth of rocket fuel, for free, over the internet.I don't plan to earn a cent from that. But you can make millions from my idea
http://www.chemicalforums.com/index.php?topic=46384.0
put for free over the Internet.
Post by: Enthalpy on August 15, 2011, 10:21:59 PM
Wouldn't dicyclobutylacetylene be better yet?
Multiple bonds aren't desired because fuels are generally used in a jacket to cool the combustion chamber, and as the fuel gets hot, its multiple bonds would let it polymerize into some gum that prevents cooling, ending the game.
Triple bonds are said to make molecules able to detonate without an oxidizer, as acetylene itself does. This is excluded in a rocket.
In the early days, fuels like NC-CC-CN (three triple bonds, no hydrogen) were tried and abandoned because of boom. And as they lacked hydrogen, they weren't even efficient.
Summing these reasons, hydrocarbon fuels tend now to be quasi-alkanes; more energetic molecules are strained but have no multiple bonds.
Post by: orgopete on August 16, 2011, 05:19:43 PM
Let's look at what has been mentioned thus far.
Quote
Triple bonds are said to make molecules able to detonate without an oxidizer, as acetylene itself does. This is excluded in a rocket.Wikipedia (http://en.wikipedia.org/wiki/Methylacetylene#Use_as_a_rocket_fuel): "…showed that propyne would be highly advantageous as a rocket fuel for craft intended for low Earth orbital operations. This conclusion was reached based upon a specific impulse expected to reach 370 s if oxygen is used as oxidizer, a high density and power density, and the moderate boiling point, which causes the chemical to present fewer problems in storage than for example a fuel that needs to be kept at extremely low temperatures."
The problem is a generalization for which I don't agree. Acetylene may pose an explosive hazard, but just as picric acid and trinitrotoluene (TNT) are explosives, nitromethane is a fuel used in dragsters and race cars, one cannot simply draw an analogy that compounds with nitro-groups are explosives. Many are and many are not. In order to distinguish, one must understand the relationship between the compounds and its reactions.
Again, remember, I am not a rocket scientist. I don't purport to have any special knowledge. In the patent Enthalpy cited, the Russian authors cited a 2% improvement over kerosene. If you research this a little further in Wikipedia, you would find the Russian space program had used a cyclopropane derivative at one time, but with the collapse of the USSR, they could no longer afford to use in in their space program. Even in the US space program, simply using a more highly purified kerosene was abandoned as too costly.
It looks as though even rocketry is limited by the laws of conservation of energy, but in an indirect way. Probably the real law is about the "bang for your buck". You can get ton quantities of kerosene by distilling crude oil. How cheap must cyclobutyl cyclobutane be in order to make a 2% improvement in performance economically favorable?
If think if Entropy can answer this question, then chemists or more likely chemical engineers can predict whether it can be done. Let me point out, it isn't a question of whether it can be done, the Russian rocket scientists already used it. The challenge is to compete with kerosene. Can you synthesize (and purify) something when the competition is distilled directly from crude oil? Want to buy a nice bridge over the East River?
Post by: Enthalpy on August 16, 2011, 11:06:14 PM
Propyne has never been used as a rocket fuel, and won't be, because it's an explosive.
One single man (Dunn) imagines it could be advantageous, and seems to be followed by Internet users. Nothing more.
Nitromethane is abandoned as a rocket fuel because it's an explosive. Pity, people hoped to have a monopropellant.
What Dragsters do in kg quantities is their business. Rockets with 1000t propellants don't take such risks.
Even N2O has exploded and killed one worker recently on a rocket.
-----
Syntin had allegedly 3 cyclopropane linked to an other, plus a pair of methyl. Rumours, not direct testimony, claim it was too expensive.
Cyclobutyl-cyclobutane has practically the same performance, and apparently cyclobutane can be isolated from crude oil. Being widely ignorant about synthesis, I imagine cyclobutane is the difficult part of the task, and fusing the rings the easier part.
-----
For a 5t payload paying 100M$ its launch, Boctane could bring 200-400kg performance more depending on how many stages can afford it - performance varies more than 2%. It means that from time to time, one payload can fly on a launcher or not. You may translate it to 5M$ more value per launch.
If 20t can be produced for 2M$ then the upper stage would use it. If 300t can be produced for 5M$ then the lower stage would use it as well.
The stuff is interesting enough that Atlas V wants to switch to it.
-----
That's a long discussion already for a question I imagined to be elementary... Let me repeat it:
Can two cyclobutane rings be linked to produce this Boctane, in 100t quantity, for a few M$?
Thanks!
Post by: Enthalpy on August 22, 2011, 12:01:43 PM
Is this too difficult?
Or is it too easy for you?
Wrong sub-forum, maybe... But I have no intention to become a chemist, so an answer obvious for everyone else would be fine to me!
Post by: fledarmus on August 22, 2011, 03:55:00 PM
Post by: Enthalpy on August 22, 2011, 05:55:37 PM
Accordingly, Russian rocket builders didn't try to make spiro molecules - though spiroheptane would bring performance indeed. Too expensive even for a very-last stage.
Boctane is to be Cyclobutyl-Cyclobutane C4H7-C4H7 (I try to attach a picture), which should be easier to produce.
Post by: orgopete on August 24, 2011, 02:53:29 AM
No answer...
Is this too difficult?
Or is it too easy for you?
Wrong sub-forum, maybe... But I have no intention to become a chemist, so an answer obvious for everyone else would be fine to me!
The challenge is the landscape. It is virtually being assumed that the Russians, Europeans, and NASA don't know what they are doing. Don't they know that bicyclobutane is a superior and cheap rocket fuel that can be readily prepared in multi-ton quantities?
From my experience, you are far more likely to be successful by utilizing something that has already been shown to work over something that even experts might predict should work but has never been demonstrated to work. Bicyclobutane is apparently a known compound based upon the patent previously cited. That seems a far better starting process than any thought of joining two cyclobutanes. My instinct is that any chemists here would suggest you can forget bicyclobutane as an alternate to kerosene on a cost basis if it is gives only a 2% improvement. However, I could be wrong. How was it prepared?
No matter, let's try it based upon hypothetical economics. Let us assume that cyclobutane can be dimerized into bicyclobutane in a single step and in 100% yield. Let us further assume cyclobutane is commercially available in ton quantities at the same price as kerosene. All that is needed is to turn the crank and we're off.
The cost to turn the crank on this process is $25/kg. That seems reasonable based upon my experience. If some of the other chemists with industrial experience can suggest another value, that is fine also. We had used a slightly higher value 15 years ago. This is where I got the $25/kg value from. http://greenchem.uoregon.edu/ACSGoingGreenSite/PDFs/20050315TuesPM/1336Cue.pdf
My experience tells me you cannot couple cyclobutane, cyclobutane is going to be far more expensive than kerosene, the known route to bicyclobutane is going to be costly in processing steps, and I respect that if Russia gave up on their cyclopropyl rocket fuel when costs had to included in their rocket program, then virtually any other hydrocarbon would have a difficult time to compete with kerosene on a cost per payload basis.
If the board were to apply the same standard to this topic, then not being a chemist would never be sufficient for not providing a shred of input. I doubt anyone thought cyclobutane could be dimerized. If cyclobutane is being asked to join together, give at least one other example or principle that would even suggest this to be a reasonable reaction. Once you do that, I think you will find a number of chemists will be happy to help you do a cost estimate from the raw material costs, reagents, reactions, and yields.
I faced this challenge working in the agchem industry. If a farmer could buy weed control for $15/acre, then if you had a herbicide that you need to apply 1 lb/acre, you need to be able to make it for $7.50/lb in order to make a profit. That will limit you to very simple compounds. However, if you only needed 0.1 lb/acre, then you could be profitable if you could prepare it for $75/lb. There is no magic to know your compound has to be extremely potent in order to compete on a cost basis. Here, the competition is crude oil. You distill it a couple of times.
Post by: Enthalpy on August 24, 2011, 07:24:17 AM
The aim isn't to be cheaper than kerosene, but more efficient. The added payload capacity is quite more than proportional to the fuel performance, and should be compared with launch charges, of which present kerosene costs are nothing.
Syntin, which contained three cyclopropane rings linked to an other, had been used. Excessive cost is suggested by one source without reference. It could just be that two professors knew how to produce it, and when Russia stopped paying their wages, one became a mammoth ivory smuggler and the other a Siberian tiger hunter. Anyway, isn't a string of C3 rings more difficult to produce than of C4?
As Atlas V plans to switch to Boctane, maybe it makes economical sense.
-----
So you consider the C4 ring will break open as soon as we near some halogen?
Post by: Enthalpy on September 01, 2011, 08:30:49 PM
Heat of formation is +80cal/g (I estimated +77cal/g)
Density 828kg/m3. Viscosity 2.4cPs @+20°C.
Melt -54.5°C / Flash +20°C / Boil +134°C / Autoig +205°C / Decomp +430°C
Post by: Honclbrif on September 01, 2011, 09:50:13 PM
1. The cost of kerosene
2. The cost of the increased payload which boctane will provide
It seems pretty easy to put a definite dollar value on how much boctane can cost and still be economical. Have you run these numbers yet?
Modification:
My reasoning is if you could double your payload with boctane, you could then charge up to double the price for kerosene for it and still count it as an economical fuel. So, can boctane be produced for double the price of kerosene?
Modification 2:
http://www.indexmundi.com/commodities/?commodity=jet-fuel, the cost of jet fuel (which as I understand it is basically kerosene) as of 8/30/2011 was about $1/kg. Assuming orgopete's estimate of $25/kg is reasonable, can boctane provide 25 times the performance?
Post by: Enthalpy on September 02, 2011, 09:34:19 AM
That is, 5% more payload capacity can be sold for 5M$. 200t of fuel for two stages, needing no hardware modification, can be bought for up to this cost. This would allow 25$/kg.
Used at the upper stage only, 40t of fuel would bring 3% more capacity, sold for 3M$, so the maximum purchase cost is 75$/kg in this smaller quantity.
Some three-stage kerosene rocket can use even smaller quantities.
This price is a gross maximum, because companies want purchases cheaper than sales, and a fuel change costs more than its purchase.
-----
Maybe I should have explained that first:
Propellants make 90% of rockets' mass, and this proportion is very hard to increase. So 5% more payload cannot be obtained by 5% more kerosene, which would have been negligible. It needs a whole rocket (100M$ recurring cost) 5% bigger, plus a new design (many G$).
In fact, a flexible approach would let most payloads fly with normal RP-1. Sometimes a payload will need just more than the launcher's capacity, and only this customer pays Boctane up to the difference between a 5t and a 10t launcher. This means smaller and irregular amounts of Boctane, but sold for much money.
-----
Rocket's RP-1 http://en.wikipedia.org/wiki/RP-1 is cheap. Not as cheap as kerosene, because components that would make bubbles or gums at heat (when it flows in the combustion chamber's cooling channels) have been removed - among several other demands.
-----
So, cost would mean, a few times a year:
- Produce two trucks of Boctane for well under 3M$, or
- Eight trucks for well under 5M$
-----
Does it make sense to start with cyclobutane, as it seems available from petroleum, and connect two rings?
I vaguely imagine it could react with a bit of chlorine diluted in nitrogen or argon, possibly at room temperature and aided with light, to produce chlorocyclobutane which must condensate more easily than cyclobutane and chlorine.
Then, I figure diluted chlorocyclobutane would be brought to react with cyclobutane - did I read AlCl3 catalyses this reaction?
Post by: azmanam on September 02, 2011, 10:59:26 AM
This paper describes such a coupling: http://dx.doi.org/10.1021/ol200617f. Probably not cost effective, but it will likely do what you want. BTW, bromocyclobutane is commercially available (though expensive). Chlorination of cyclobutane is probably not good, due to frequent polyhalogenation. AlCl3 does couple alkyl groups... but only to aromatic rings. That is, one of the starting materials must be an aromatic ring (like benzene). It won't couple alkyl halides to cyclic alkanes.
Step 1: cyclobutane (however you obtain pure cyclobutane in bulk) + Br2 + light/heat --> bromocyclobutane
Step 2: bromocyclobutane (2 eq) + {conditions in linked paper} --> boctane
Step 3: Fly me to the moon (in other words)
ps, I want in on the patent :)
Post by: fledarmus on September 02, 2011, 11:23:05 AM
Post by: Enthalpy on September 02, 2011, 08:27:59 PM
I believe the unsubstituted cyclobutane is made from irradiating ethylene with UV lightHope to understand it properly:
http://classes.yale.edu/chem225b/studyaids/pericyclic/pericyclic.htm
Ethylene is first excited by a photon around 171nm, and then it accepts to couple with an other ethylene to form cyclobutane.
And then I imagined to have some 1,3-butadiene vapour diluted in ethylene, and to excite butadiene around 217nm so it reacts with ethylene and forms Boctane...
But 6eV UV light must first be produced! I put some figures on the output power of 100,000 blue LED from DVD burners, tripling the photon energy, and got nothing at the end. 1kg/day if all light served :'(
Other attempt with a deuterium arc lamp, the L1314 or L1835 by Hamamatsu:
http://jp.hamamatsu.com/resources/products/etd/pdf/L1314_L1835_TLSO1012E04.pdf
inject 150W electricity, obtain 15µW/cm2 UV under 230nm... :'( :'(
With 100% energy efficiency from the mains to ethylene excited at 7eV, 1kg of cyclobutane would cost "only" 3.3kWh - still affordable, but not with efficiencies like 5% (Led+tripler) or 4ppm (Deuterium lamp), of which only a part is absorbed...
I've always had a bad feeling about photochemistry in ton amounts. It works if you have free Sunlight over hectares, and converters that multiply spontaneously for free.
Post by: Enthalpy on September 02, 2011, 08:58:40 PM
...reductive coupling of alkyl halides...challenging...This paper describes such a coupling: http://dx.doi.org/10.1021/ol200617f. Probably not cost effective...Chlorination of cyclobutane is probably not good...AlCl3 does couple alkyl groups... won't couple alkyl halides to cyclic alkanes.
Oops, the paper wants such things, which indeed don't look cheap
http://en.wikipedia.org/wiki/File:Ni%28cod%292.png
http://en.wikipedia.org/wiki/File:PyBOX.png
Chlorination: I had hoped dilution would prevent multiple halogenations, and to begin with, let the reaction make Pshhhhh instead of Boom. But I have absolutely nothing against bromine! Maybe the undesired HX elimination leading to a double bond is less frequent with halogeno-cyclobutane, as cyclobutene is energetically unfavourable.
Step 3: Fly me to the moon (in other words)
ps, I want in on the patent :)
Too late for a patent! Patentable material must be new, which means, unpublished. In other words, I don't seek a patent.
But the Moon, sure! :) With present technology, a trip equivalent to the Apollo mission would fit in this launcher:
http://saposjoint.net/Forum/viewtopic.php?f=66&t=2554&start=20#p30805
it's the spaceship underprice.
Post by: Enthalpy on September 06, 2011, 12:41:47 PM
Agip is rumoured to have dissolved some Cubane in a competition gasoline before it was forbidden or detected, so at least some 10kg can be made presently, for a few M$. Still some way to go maybe?
And if Cyclobutane dissolves Cubane in good amounts, it could improve an already excellent propellant, especially if this keeps Cyclobutane liquid to a higher temperature.
Post by: Enthalpy on September 15, 2011, 10:02:08 AM
For instance a low-pressure mercury lamp produces 90% of its light at 254nm:
http://www.photochemicalreactors.co.uk/immersion.htm
from these figures, power efficiency is 13% at this wavelength.
254nm is short of butadiene's broad 217nm absorption peak, but other sources (zinc?) may fit better.
By the way, I'm not sure ethylene would fuse with butadiene at the end instead of the centre, as the two double bonds aren't local. But if butadiene (or hexatriene, fits 254nm) wants to produce a ladderane
http://en.wikipedia.org/wiki/Ladderane
I have absolutely nothing against it, of course...
Post by: Enthalpy on February 07, 2012, 09:22:34 PM
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv5p0528
with a good material efficiency, and using a 450W lamp for 72h to obtain 100g.
(a) No ladderane was obtained, though a little bird tells me they tried.
(b) A medium-pressure Hg lamp is 7% efficient, and each produced molecule needed 26 photons.
http://www.photochemicalreactors.co.uk/immersion.htm
(c) This means 0.5M€ electricity for 10t of fuel, still acceptable for an upper stage.
In mass-production, I suppose they would irradiate less and separate earlier the product from the reactants, saving light.
In addition, Divinyl-Cyclobutane is available industrially (cas=6553-48-6) and some cheaper synthesis may exist.
(d) I naively imagine Divinyl-Cyclobutane can further react with Ethylene under UV light to obtain trans-1,2-Dicyclobutyl-Cyclobutane
(e) Divinyl-Cyclobutane and other unsaturated compounds aren't wanted in a rocket fuel. But if Dicyclobutyl-Cyclobutane dissolves in Dicyclobutane and widens its liquid range, it would be welcome - in addition to Cyclobutane and maybe Cubane.
Comments please?
Post by: Enthalpy on July 06, 2012, 03:04:22 PM
Now, at an existing stage, a denser fuel can keep the tanks and the turbo-pump (but not the throat, the nozzle... neglected here). The rocket weighs more (who cares) but the engine pushes proportionally more and keeps all pressures as it pumps the same volume (yes, one pump is slightly mismatched).
Here I keep the fuel's volume, a big handicap for amines. It's re-optimized once for RP-1 instead of the Russian RG-1. The RP-1' heat of formation here is unfair but I compare the other fuels anyway.
In the following table (log in to see it), Deta is DiEthyleneTriAmine, tm-triazinane is 1,3,5-trimethyl-hexahydro-triazine, Teda is TriEthyleneDiAmine and Hexamine is Hexamethylene Tetramine. The latter two are solids but I want to dissolve some in an amine to densify it.
While Boctane is good, dirt-cheap cis-Pinane and Deta are as good, and the common missile fuel JP-10 even better here. Even 10% or 20% of dissolved Teda or Hexamine should improve further. These chemicals are already mass-produced.
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on January 11, 2013, 12:35:30 PM
but several better books about oil tell clearly that no cyclopropane nor cyclobutane was found in crude oil, even naphthenic. I've been naive, sorry.
Then, fusing two plain cyclobutane to produce Boctane looks like a bad idea, since the synthesis of cyclobutane in tons amount seems to be as difficult, or even more, than other compounds like methylene-cyclobutane or dimethylene-cyclobutane, which lead to propellants at least as interesting as Boctane.
So without producing first cyclobutane:
Either optimize photochemical synthesis on the big scale. Use butadiene and ethylene to get a mix of vinyl-cyclobutane and divinyl-cyclobutane and maybe cyclobutane, then a welcome mix of cyclobutyl-cyclobutane (Boctane) and dicyclobutyl-cyclobutane and possibly cyclobutane;
Or save the thousands of lamps, go some cyclobutane-related route like methylene-cyclobutane derivatives, obtained from allenes and alkenes, and produce better fuels than Boctane, like spirohexane or dispirooctane derivatives. To my untrained eyes, methods described in the old US patent 4,031,152 look usable for tons or products. Or aren't they?
Unless, of course, the inventors of Boctane as a rocket fuel have a better production method.
These hydrocarbons are only for cases where amines aren't acceptable.
Post by: curiouscat on January 11, 2013, 02:21:50 PM
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv6p0133
How to make 1-bromo-3-chlorocyclobutane I've no clue!
Post by: Enthalpy on January 11, 2013, 11:28:13 PM
http://www.youtube.com/watch?v=WsVzpE7ltb8
this one was a solid booster. Liquids make a shorter but more intense show.
And because rocket engineers find this less fun than a Buster Keaton movie, they exclude propellants like hydrogen peroxide or nitromethane that are harmless in gram quantity.
Accordingly, Boctane is cyclobutyl-cyclobutane, or H7C4-C4H7. Two C4 rings linked.
Plain Cyclopropane is about the limit of stability. Cubane would be perfect if scalable and affordable. A Russian company develops acetylene, but diluted in ammonia - hope its' stable, yuk anyway.
Post by: curiouscat on January 11, 2013, 11:34:28 PM
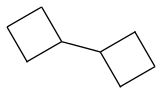
This is the one you want to make?
Post by: Enthalpy on January 13, 2013, 06:38:21 AM
The description of an attempted photosynthesis from butadiene and ethylene is coming.
Post by: Enthalpy on January 13, 2013, 09:14:44 AM
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on January 13, 2013, 01:10:40 PM
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv5p0528
and despite having no experience for it, I dared to adapt the idea, so please take my proposal with all due caution.
Photoaddition is praised to avoid cyclodimers, like cyclooctadiene from butadiene. Further, to let reactants add with one of a different kind, I imagine to excite only one reactant through wavelength selectivity, and have the excited reactant less concentrated so it combines more probably with a different reactant, instead of dimerizing.
Excimer lamps exist with proper wavelengths. See for instance Iupac, Heraeus or others:
http://old.iupac.org/goldbook/ET07372.pdf
http://www.heraeus-noblelight.com/en/products_1/uvprozesstechnik_1/uvp_excimer.aspx
- Xe2 radiates at 172nm, 900W consumption, perfect for ethylene;
- KrCl at 222nm, 3000W consumption, fits 1,3-butadiene around 217nm;
- KrI 190nm, ArF 193nm and others are not catalogue parts.
Xe2 is said to offer up to 40% efficiency, good others 5 to 15%.
I plan no sensitizer, since the lamps have the proper wavelength. This shall hopefully improve the light efficiency; the excimer lamps would then consume less electricity than 50k€/t used by the 7% efficient mercury lamp in the above cited photoaddition, which has 1/26 quantum efficiency. Stirring and permanent reactant feed shall improve also. Would a sensitizer improve or degrade the reaction selectivity? No idea. Maybe a wrong choice, or not.
Solvents widen too much the absorption wavelength peak, so gases shall react. With light absorption of 15000/cm and 21000/cm for ethylene and butadiene in molar solution, 1mb of reactants would already absorb within 1cm, so stir if using a higher partial pressure. The reactants can hence be cold, nice against spontaneous polymerisation and leaks; operating near their (reduced pressure's) boiling point would let the products condense away as they form. To widen absorption peaks by 2*5nm and fit the lamps, add about 10b of a nonreactive gas (nitrogen? Rare gas?) which must be pure for transparency. Mind absorption throughout light path!
----- To vinyl-cyclobutane
Excited butadiene reacts mainly with the more abundent ethylene to make vinyl-cyclobutane; proportions can keep some divinyl-cyclobutane.
Alternately, excited ethylene reacts mainly with the more abundent vinyl-cyclobutane. This would produce more cyclobutane, which shall be controlled in a rocket fuel as it's volatile, so distill. Products separation within the reactor also gets less obvious.
----- To cyclobutyl-cyclobutane (Boctane)
Excited vinyl-cyclobutane (by KrI, ArF lamp?) reacts mainly with the more abundent ethylene to make cyclobutyl-cyclobutane (Boctane).
Alternately, excited ethylene reacts mainly with the more abundent vinyl-cyclobutane. This would produce more cyclobutane.
The 1,2-trans-divinyl-cyclobutane fraction makes dicyclobutyl-cyclobutane, which is denser than Boctane, less flammable, and could well improve the melting point, so keep it in Boctane! Adjust its proportion through the gas partial pressures in the first cycloaddition.
Photo dimerization of vinyl-cyclobutane will also provide some dicyclobutyl-cyclobutane, possibly the 1,3 isomer whose mix should lower the melting point, welcome. Tune the gas partial pressures in the second cycloaddition.
----- Variants
I wish C=CC=C butadiene is replaced by piperylene C=CC=CC, isoprene C=C(C)C=C or an other, or some mix. The product should offer a lower melting point, a higher flash point, and the reactants are less volatile if not more healthy and stable. Store cold. Ethylene also can be replaced or mixed. A blended product, from mixed reactants or through successive batches or both, uses to offer a lower melting point and viscosity.
Is the (substituted) dicyclobutyl-cyclobutane alone better than Boctane? Denser and less flammable, but what melting point? It should be feasible from divinyl-cyclobutane (see Orgsyn's paper) and ethylene, or (1,3-?) from vinyl-cyclobutane. Or start from 3-methyl-hexatriene, available from rubber pyrolysis, which seems to cyclise less than hexatriene, and could have been the reactant to Syntin. Prefer blends as usual.
Divinyl-cyclobutane could be cyclopropaned instead. Isomer of the old Syntin, same performance. Substitute and blend.
More distant enes in the reactant would make a product more flexible and unsymmetric than Boctane. Less dense, but lower melting point. If the melting point improves enough, a third ring would regain density.
A bit of cyclobutane is acceptable in the fuel (cubane as well, thanks); excess might be sold, reverted to ethylene (in real time?), converted separately to Boctane if possible. Cyclobutene should be stripped and reverted to butadiene.
Xe2 lamps to produce cyclobutane could be interesting by themselves, even if not to make Boctane. It could even be possible on Mars; cyclobutane needs less hydrogen brought there than to produce methane from the atmosphere - though I'd instead burn hydrogen with local oxygen, or rather bring the propellants.
If exciting only ethylene, both steps might occur in the same reactor... Or if providing light at both wavelengths for butadiene and vinyl-cyclobutane... Then, vinyl-cyclobutane must be gaseous and cyclobutyl-cyclobutane better liquid. Mind cyclobutane amount as well. Less easy to tune!
The inspiring paper at Orgsyn excites Michler's ketone around 336nm from medium-pressure mercury. Usable for vinyl-cyclobutane? Optimum sunlight brings 4W/m2 between 336nm and 306nm.
Comments welcome!
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on January 14, 2013, 06:03:38 PM
Because ethylene and butadiene give a "good yield" of vinyl-cyclobutane over titanium catalyst.
And butadiene dimerizes to cis-1,2-divinyl-cyclobutane over nickel(0) catalyst.
From http://onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.1973.tb49527.x/abstract
"The catalyzed codimerization of ethylene and butadiene to vinylcyclobutane" by Lawrence G. Cannell
So at least the first step needs no UV light.
In case the second step is a cyclopropanation instead, it needs no light neither.
Excimer lamps are recent. Most literature refers to mercury lamps, at medium (wide 336nm) or low (narrow 254nm) pressure, which means excitation of most molecules had to be indirect. Excimer lamps could change the reactions' selectivity and photon efficiency - or it's wishful thinking.
Post by: Enthalpy on January 14, 2013, 08:08:17 PM
Textbooks don't reveal one thing... If you know the yields, please tell! The amounts of light would be interesting also.
Cyclobutene appears to live long enough at room temperature, reverting to butadiene around 175°C rather. Though, polymerization competes with the ladderane. Dilute and chill? It looks risky at industrial scale.
Post by: curiouscat on January 16, 2013, 04:15:13 AM
Dilute and chill? It looks risky at industrial scale.
Why?
Post by: Enthalpy on January 16, 2013, 10:28:31 PM
Cyclobutene seems to polymerize willingly and once this heats it, can release energy when transforming into varied things. Similar to the risk of butadiene; cyclobutene might be less prone to polymerization than a conjugated diene, but it releases more heat once it decomposes.
Well, it's a matter of amounts... Producing 200t in 20 days can mean big batches, but a continuous process would minimize the amounts at the plant.
I've found no safety data about ladderanes. Gram amounts are big news, so their risks can't be well known.
By the way, a rather recent ladderane production method holds two identical molecules close together, each with a C=C-C chain, and irradiates them with UV. If one could make the same with two (substituted) benzene rings to produce hexaprismane, it would bring 2/3 of cubane's advantage as a rocket fuel... The evenly spread strain of hexaprismane may (?) give it stability, and perhaps it's less difficult to produce than cubane.
A lone excited benzene ring is known to isomerize, supposedly before reaching the next benzene since no hexaprismane was reported from these experiments, thus the two stacked rings. Six new bonds may also require an excited level that is not the lowest one. And as a wild extrapolation, substitutions at different positions on both rings may favour the required excited state.
Marc Schaefer, aka Enthalpy
Post by: curiouscat on January 16, 2013, 11:00:56 PM
Quote
If one could make the same with two (substituted) benzene rings to produce hexaprismane, it would bring 2/3 of cubane's advantage as a rocket fuel.
Hmm....I didn't understand how you calculated that. Advantage = energy capacity?
Post by: Enthalpy on January 17, 2013, 12:32:23 PM
http://www.propulsion-analysis.com/
as it depends on the elemental composition (more hydrogen is better because H2O is light), the isentropic coefficient (N2 and CO convert better the pressure ratio into kinetic energy), and the heat of formation of the fuel (less hydrogen for more strain; multiple bonds are unwanted).
Alas, I have only AM1 and the like to estimate the fuels' heat of formation as a liquid at RT, and have nearly zero confidence in it, hence the voluntarily vague estimate of "two-third". Pure cubane (solid if not substituted) would improve the gas ejection speed by some 180m/s over kerosene and hexaprismane by some 120m/s, but this is very inaccurate.
120m/s (over 3300m/s for kerosene) isn't a game changer. Only hydrogen (4300m/s) makes a dramatic difference with hydrocarbons. But in existing designs, Boctane (+50m/s) gains like 8% payload in orbit, for which people want to pay. 120m/s would be an interesting improvement even for a new rocket design, not just a retrofit.
Though, hazards and some nuisances would let me give away 120m/s improvement:
(1) Explosion. A launcher has 800t of propellants. Nitromethane is abandoned, hydrogen peroxide nearly so, even N2O has killed a worker recently.
(2) Toxicity. MMH would bring performance but everyone wants rid of it.
(3) Fire. Kerosene has +55°C flash point, I wouldn't take cyclopropane for 100m/s more. Boctane at +20°C isn't perfect.
(4) Hypergolic ignition is often told as an advantage... But we have igniters for that. Leaks igniting is a serious drawback of amines if nitrogen tetroxide must be used somewhere.
(5) Melting point and viscosity, depending on where the propellant goes... Mars.
(6) Price, yes.
A really funny narrative by an early developer of rocket propellants:
Ignition! An Informal History of Liquid Rocket Propellants, by John D. Clark
http://library.sciencemadness.org/library/books/ignition.pdf
these times are over. Presently, we have solids, and hydrazine/N2O4 being phased out, and oxygen combined with hydrogen or RP1 (special kerosene). Boctane is one attempt to improve over kerosene and keep compatibility.
Post by: Enthalpy on January 22, 2013, 07:05:36 PM
How an ignorant like me imagines it (log in to see the sketch below):
- Use a UV wavelength absorbed by cyclobutene directly, without a sensitizer, and not by acetylene.
- Excimer lamps exist now and give choice for the wavelength: better than mercury.
- Put both cyclobutene and acetylene at moderate partial pressure, so light makes a reasonable path. Or stir well. Or both!
- Have some clean non-reacting gas (Ar? N2?) at the proper pressure (10b?) to broaden cyclobutene's absorption and include the lamp's wavelength.
- Adjust the cyclobutene-to-acetylene ratio to drive the mean length of the ladderane.
- Alternately, choose the temperature and pressure so that the desired half-length ladderene condenses, and (co-) dimerize it in a later UV step.
Longer ladderanes would near the benefit of hexaprismane as a rocket fuel: far better than Boctane, 2/3 the benefit of cubane. And anyway, more people would like to have some, first as an exotic toy, and later they may find uses.
Marc Schaefer, aka Enthalpy
Post by: discodermolide on January 23, 2013, 02:50:11 AM
Post by: Enthalpy on January 23, 2013, 06:55:57 PM
They make governments and patent attorneys richer, and inventors poorer as a mean result. Protection in the UE, US and Japan (China?) would cost many k€ initially, and more every year, for each invention.
A patent gives the inventor only a means of proof. Once a multinational company has decided to infringe your rights, you still have to win a legal action against them.
My employers patented some of my inventions - but not the RFID! - and earned no cent through these patents. Individual inventors who can make companies pay for ideas, like Roland Moreno did, are even less common.
As a result, I prefer to give away for free the ideas I won't develop by myself.
Worse: I understand approximately nothing from chemistry, so most of my ideas here, especially synthesis routes, must be nonsense. Luck helped other people for the production of fullerenes, but miracles are scarce. Chances are better at other ideas, like the quick production of ice, the reactor with rotating disks, the electrostatic alternator, the extruded rocket tanks, the transcranial magnetic stimulation...
Good luck to people willing to try them!
Post by: fledarmus on January 23, 2013, 08:40:26 PM
The idea that having a good idea and rushing out to get a patent on it will be the key to wealth is seriously overblown. A successful inventor must be willing to carry an invention through from idea to prototype, to commercial embodiment, to marketing, and in the meantime to work on improvements and new inventions. Getting a patent along the way is essential to keep the next person from simply looking at all your hard work and saying "I can do that", without going through all the hard work of development. A company that is truly interested in product development rather than copying will usually be able to design their way around your invention if the solution is really important to them, and if your patent isn't something they are already working on, there is very little incentive to develop it if you haven't already done all the legwork to make it productive.
Post by: curiouscat on January 23, 2013, 10:19:53 PM
Worse: I understand approximately nothing from chemistry, so most of my ideas here, especially synthesis routes, must be nonsense. Luck helped other people for the production of fullerenes, but miracles are scarce. Chances are better at other ideas, like the quick production of ice, the reactor with rotating disks, the electrostatic alternator, the extruded rocket tanks, the transcranial magnetic stimulation...
Do you keep them as ideas or have you built prototypes for any? e.g. the rotating disc reactor I'd be interested in seeing how it operates.
Post by: discodermolide on January 23, 2013, 11:23:35 PM
[/quote]
I find it difficult to believe that you know nothing about chemistry, even approximately. Some of the reactions you have presented to us may just be feasible so why not try them out experimentally? This because chemistry is an experimental science and you never know until you try!
On the other hand by publishing your ideas here you are preventing others, who may be thinking along similar lines, from being able to patent their ideas (I am thinking more of companies here, not individuals).
Also I find it strange that, as you said you understand "approximately nothing from chemistry" , you are posting these ideas at all. The object of this forum, apparently, is to educate those who have difficulties with the subject, so what are you learning?
Post by: Enthalpy on January 27, 2013, 11:41:12 AM
Hence what I suggest on Internet forums is more a message in a box, available for everyone, generally untested. My proposals for electrical and mechanical engineering are likely to work, but for chemistry not likely.
Try by myself: I have nothing here! I came with my backpack and got my computer meanwhile.
I keep a few other ideas for me, which I can develop by myself with an investment accessible to an individual, and which don't compete with Exxon-Apple-etc.
Post by: Enthalpy on January 28, 2013, 08:01:47 AM
Strength depends on the material... Sugar for instance can make big solid parts that don't break. Single-crystals also differ from polycrustalline or amorphous materials; they're often much stronger but brittle.
Post by: discodermolide on January 28, 2013, 09:46:40 AM
Silicon boule are hold at their seed only because of their outstanding strength. As soon as the final diameter is grown from the seed, a normal material can be grasped there.
Strength depends on the material... Sugar for instance can make big solid parts that don't break. Single-crystals also differ from polycrustalline or amorphous materials; they're often much stronger but brittle.
I think you placed this in the wrong place?
Anyway I was talking about polymorphs of any particular crystal. If you generate a seed from one process it will have morphology , let's say A. It may be meta-stable and when you place it in your solution to grow it may easily revert to a different morphology, say B. Now if the compound you are interested in has morphology A then you are royally screwed. This mainly because all the tests and evaluations you have done, even registration has been done with A, if you suddenly produce B then the whole lot must be repeated. You may even find different bio-availabilities,rates of dissolution and the like. So the whole process of crystal growth is a minefield. Which is why Pharma companies (I can only speak for them) have crystal engineering departments.
Post by: curiouscat on January 28, 2013, 09:53:11 AM
On a flight of fancy, I was wondering if one could make a machine with a high resolution camera and a nanofluidic channel that'd sort individual crystals as they went by?
Something kinda like a flow cytometry cell sorter. Or are these crystals too small to be able to do that?
Post by: discodermolide on January 28, 2013, 10:03:01 AM
As you probably know this is how phase separations are done in the plant, using refractive index changes at the solvent(s) interface.
Post by: curiouscat on January 28, 2013, 10:19:49 AM
As you probably know this is how phase separations are done in the plant, using refractive index changes at the solvent(s) interface.
Yep. But that sounds a lot easier. RI is a macroscopic property needing only a sensor.
I was thinking of crystal detection with some real time image processing algorithm. Although now that you mention is just an optical rotation sensor might suffice?
Post by: discodermolide on January 28, 2013, 10:50:36 AM
Optical rotation is ok if it's large enough, what happens when the rotation is very small? Then you usually measure at different wavelengths. And the rotation depends upon the solvent you use for the measurement.
So maybe your first idea is better?
Post by: Enthalpy on February 04, 2013, 10:12:15 AM
So sorry! :-[
Post by: discodermolide on February 09, 2013, 10:44:30 AM
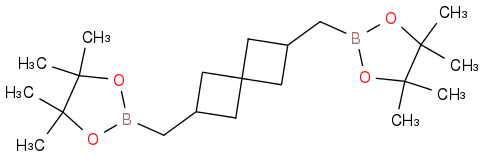
It appeared in the Journal of the American Chemical Society 2013, doi.org/10.1021/ja3104582 along with a host of other compounds of interest to you. The coupling method is especially nice.
Post by: Enthalpy on February 10, 2013, 06:54:17 PM
Boron is advantageous if the compound is affordable and not toxic. LiBH4 is one compound that would improve performance over hydrocarbons.
But... Would you have the same one without oxygen? I'd accept a different colour and smell if needed.
Post by: discodermolide on February 10, 2013, 07:08:41 PM
Post by: Enthalpy on February 15, 2013, 10:05:02 PM
A rather recent ladderane production method holds two identical molecules close together, each with a C=C-C chain, and irradiates them with UV. If one could make the same with two (substituted) benzene rings to produce hexaprismane, it would bring 2/3 of cubane's advantage as a rocket fuel...
Hexaprismane was nonsense :P - and nobody tells me a clear word... >:D
- Cubane was the first prismane to be synthetized, just to picture the difficulty.
- Strain energy is slightly less in pentaprismane (which has been synthesized) than cubane but worse in hexaprismane - which has not been seen up to now.
- Aromatic rings don't stack willingly, quite the opposite.
- I had hoped to hold by carbon chains two benzene stacked. It's banal and called a cyclophane
http://www.org-chem.org/yuuki/cyclophane/cp_en.html
but if a [2.2]cyclophane gave a hexaprismane, this one would include [2.2.2]propellanes which are unstable, and a longer cyclophane is bad as a rocket fuel precursor.
- Guess what, many people tried precisely that and haven't succeeded up to now
http://sciencelinks.jp/j-east/article/200415/000020041504A0465587.php
including by irradiation, after which the compounds rearrange a lot.
I've also vaguely tried to run a software (hence unreliable) on metal-cyclopentadiene and metal-cyclooctatetraene sandwiches, and PM3 claims that it is not a way to bring two C5 or C8 rings (flat aromatic anions) closely stacked, neither within a single complex nor between two complexes.
Post by: curiouscat on February 15, 2013, 10:32:45 PM
A rather recent ladderane production method holds two identical molecules close together, each with a C=C-C chain, and irradiates them with UV. If one could make the same with two (substituted) benzene rings to produce hexaprismane, it would bring 2/3 of cubane's advantage as a rocket fuel...
Hexaprismane was nonsense :P - and nobody tells me a clear word... >:D
....and who wrote that nonsense?
Post by: Enthalpy on February 15, 2013, 11:27:44 PM
Just for fun:
In the solid, benzene rings arrange rim-to-face if I understand properly. A pressure like 15,000 bar (still accessible to steel cylinders and pistons) should bring them parallel, to the same 300pm distance as in a [2.2]cyclophane, ready for photoexcitation, but flat and without the [2.2] links that bring unstable propellanes in the desired prismane and introduce rearrangement paths.
Provided the benzene rings really get parallel, they wouldn't necessarily be aligned, but maybe some substitutions bulkier than in xylene would help - or take the [3.3]cyclophane and compress it to the distance of the [2.2].
Then, irradiate through the ceramic piston: if the volume shrinks you're on the right path, but if the cylindre detonates you better have stayed out of the path.
Well, the pressure relies on energy computations by AM1, hence suspect... All by a non-chemist, even more suspect.
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on August 08, 2015, 08:10:02 AM
http://www.chemicalforums.com/index.php?topic=50579.msg233818#msg233818
In the alkenes-to-cyclobutane photoaddition, an excited electron is said to spread to both alkenes when they meet. Exciting only the bigger alkene diluted in ethylene would be more selective, but meanwhile I doubt that the excited electron from the bigger alkene has enough energy to spread to the smaller one.
The photoaddition remains possible, with an Xe2 lamp at 172nm exciting the ethylene. It will also excite the vinylcyclobutane (represented by vinylisopropane in the appended spectra), so the partial pressures can't differ too much, and the reaction is less selective, giving as well the useful tricyclobutyl and byproduct cyclobutane - see the appended sketch.
Let's imagine an equimolar mix, equal cross-sections and equal cycloaddition probabilities. Four absorbed photon moles would yield 164+110g of products and waste only 56g ethylene. If the lamp is 40% efficient and the reactions 50%, 1kg fuel needs 29 mol photons and affordable 14kWh.
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on March 20, 2016, 05:06:01 PM
"Science of Synthesis: Houben-Weyl Methods of Molecular Transformations" ch. 48.3.1.2
(from 1,4-dichlorobutane and gaseous K+Na at 220°C and 0.1 torr with 70% yield), so it's interesting again to "oligomerize" cyclobutane.
The C-H dissociation takes 404kJ/mol for cyclobutane as for tertiary unstrained alkane, according to
Neumann, "Organic Chemistry", chap 11.4
which is said to ease free-radical halogenations. Though, I haven't found direct reports of cyclobutane halogenation. Comments welcome!
Free-radical halogenations may exceed the intended degree, but
- I want anyway a mix of bis- and tris-cyclobutyl (appended sketch) to raise the flash point from +20°C to +55°C
- Plentiful isomers are welcome to lower the melting point
- If some isomers freeze too easily, they can be filtered out at cold
- I plan to separate the products as the reactor synthesizes them.
Since unselective halogenation is fine, maybe Cl2, HBrO, Br2+Cl2 or even H2O2 can accelerate this step and save UV light further. Could the reaction rate be controlled by slowing the halogen inflow?
The oligomers shall result from Wurtz coupling (or a better idea).
- The competing cyclobutene production is energetically more defavoured than with straight alkanes.
- More than three rings aren't supposedly desired as they freeze more easily. They can be distilled or freezed away.
- Bis- and tris- simultaneously maximizes the proportion of monohalocyclobutane in the reactor hence reduces the too heavy products.
- What if the mono and dihalo bear different halogens? I imagine more reactive dibromocyclobutane kept in excess chlorocyclobutane by controlling the inflow in the continuous process. Opinions?
- Maybe the metal can be gaseous if advantageous.
How easy would it be to couple a halocyclobutane with plain cyclobutane? For one lorry a week of course. Avoiding the metal would be nice, and hopefully the degree of oligomerization is easier to control.
Details should come about the reactor that separates the mono-, di-... halocyclobutanes.
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on March 24, 2016, 04:03:16 PM
The other way may be better then: have a more reactive (bromo) monohalo- and less reactive (chloro) dihalo- cyclobutane, and enrich the reaction zone with dihalo to get the desired proportion of tris- versus bis- cyclobutane.
Post by: discodermolide on March 25, 2016, 06:01:57 AM
Post by: Enthalpy on March 25, 2016, 07:01:04 AM
I'll try to find literature and see if I grasp something...
Post by: Enthalpy on March 25, 2016, 07:40:26 PM
It's straightforward and usable for other compounds, so it must exist already. Books must refer to lab experiments, not to a continuous process, when they tell that the degree of halogenation is difficult to control.
Here X2 could also be a hypochlorite, a mix of halogens, or H2O2 in case it works
http://www.chemicalforums.com/index.php?topic=80085.msg296423#msg296423
A slow influx of halogen or equivalent may help control the quickest ones, a bit of added Br2 or I2 too. Some halogens need cooling rather than permanent heating. Not represented neither is the evacuation of HX.
The distillation can also use a packed column. To harvest only one degree of halogenation, a single reactor that condenses that one suffices. Two or more reactors permit to scoop both the mono- and dihalo- here, where independent pressures let adjust the temperature. Separated reactors can also use different halogens.
1atm boiling points in °C estimated by Mpbpvp:
Cl Br OH
+13 +13 +13 C4H8 cyclobutane
+81 +108 +112 C4H7X
+125 +174 +182 C4H6X2
+159 +232 +242 C4H5X3
Well separated.
Marc Schaefer, aka Enthalpy
Post by: Enthalpy on May 30, 2018, 05:01:01 PM
Novel Metathesis Chemistry, edited by Y. Imamoglu, L. Bencze
page 336 and around (excerpts on Google Books).
Metathesis of easily obtained methylenecyclobutane (optionally with dimethylenecyclobutane), followed by hydrogenation, give dicyclobutyl (and tricyclobutyl), as sketched here. The desirable mix of both is supposedly even easier to get. Heavier compounds don't hurt in small amount and are easily separated.
Caution: the performance table in the book isn't credible. Maybe their "kerosene" reference is very bad, but dicyclobutyl gains only 4s over the usual RG-1, and the spiro compounds are denser and more efficient than the cyclobutyls. Biased comparison.
For safety (and density), tricyclobutyl is better, or at least a mix. Then, I prefer a 2+2 photocycloaddition of vinylcyclobutane as on August 08, 2015 if it works. If correct, the data for linear tricyclobutyl is already very pleasant: bp+190°C, mp-47°C, 890kg/m3, just half a second behind dicyclobutyl.