Post by: Calicum on January 17, 2013, 12:00:48 AM
Post by: discodermolide on January 17, 2013, 12:42:28 AM
Trans double bond, cis double bond?
And so on.
If we do not know what you are talking about then we can't answer the question.
Post by: Calicum on January 17, 2013, 12:47:25 AM
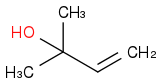
Post by: discodermolide on January 17, 2013, 12:48:55 AM
Post by: Calicum on January 17, 2013, 12:52:42 AM
Post by: discodermolide on January 17, 2013, 12:54:26 AM
Post by: Calicum on January 17, 2013, 01:04:10 AM
I kind of fear this is the major product:
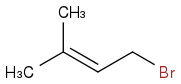
Maybe if I do it at 0o Celsius I will get this?:
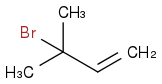
Post by: discodermolide on January 17, 2013, 01:11:23 AM
You may have to go much lower than 0°C to start seeing temperature effects.