Post by: neobenzene on January 19, 2013, 10:11:06 AM
Thanks
Post by: discodermolide on January 19, 2013, 10:24:22 AM
Post by: neobenzene on January 19, 2013, 10:31:02 AM
So in that case I'll get the following compound.....is that right?
Post by: curiouscat on January 19, 2013, 10:38:06 AM
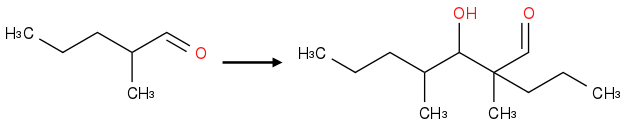
Post by: neobenzene on January 19, 2013, 10:39:55 AM
I didn't understand what you did....
Post by: discodermolide on January 19, 2013, 10:41:20 AM
Please put your pictures in a readable form, i.e. for English speaking from left to right.
For Australians upside down, etc.
He used the Smiles engine!
Post by: neobenzene on January 19, 2013, 10:44:26 AM
Thanks for the *delete me*
Post by: curiouscat on January 19, 2013, 10:46:53 AM
Haha, sorry about that! I forgot to rotate the image after scanning it.
Thanks for the *delete me*
You can fix it now.
Post by: neobenzene on January 19, 2013, 10:48:45 AM
Post by: discodermolide on January 19, 2013, 10:50:41 AM
Post by: neobenzene on January 19, 2013, 10:52:17 AM
Post by: curiouscat on January 19, 2013, 10:53:31 AM
Huh???
Try search.
Post by: neobenzene on January 19, 2013, 10:55:57 AM
http://www.prophecytimes.com/default.aspx?ctl=ctl_forum_topics.ascx&ID=5371
Post by: discodermolide on January 19, 2013, 11:00:14 AM
http://www.chemicalforums.com/index.php?topic=34153.msg130861#msg130861 (http://www.chemicalforums.com/index.php?topic=34153.msg130861#msg130861)
Post by: neobenzene on January 19, 2013, 11:02:54 AM
Thanks for the enlightenment! ;D
Post by: orgopete on January 19, 2013, 11:25:35 PM
I'd like to see a reference for tis reaction. I don't think you can form his wih NaOH.
Post by: curiouscat on January 20, 2013, 12:13:26 AM
I'd like to see a reference for tis reaction. I don't think you can form his wih NaOH.
No clue at all. You might be right.
I was only typesetting his hard-to-read handwritten equations.
Post by: souro10 on January 20, 2013, 01:49:39 AM
I'd like to see a reference for tis reaction. I don't think you can form his wih NaOH.
Why not? Which step? The aldol step or the condensation step?
I can't think of any objection one could have with the aldol step.
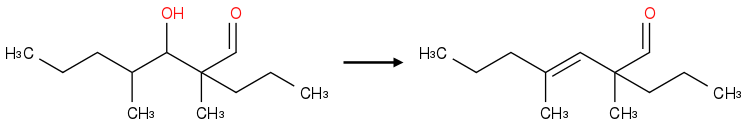
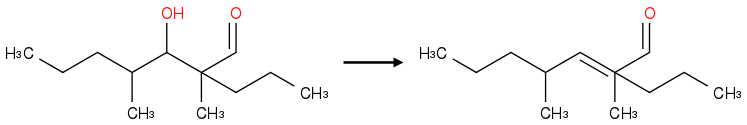
The condensation product is wrong though. The base will pull a proton from the most acidic (next to the carbonyl group, in this case there's only one such carbon ) source, leading to enolization, double bond migration, and loss of hydroxyl group to produce an alpha-beta unsaturated aldehyde.
Post by: curiouscat on January 20, 2013, 02:16:52 AM
I'd like to see a reference for tis reaction. I don't think you can form his wih NaOH.
The base will pull a proton from the most acidic (next to the carbonyl group, in this case there's only one such carbon ) source, leading to enolization, double bond migration, and loss of hydroxyl group to produce an alpha-beta unsaturated aldehyde.
This?
Post by: souro10 on January 20, 2013, 02:11:50 PM
I'd like to see a reference for tis reaction. I don't think you can form his wih NaOH.
The base will pull a proton from the most acidic (next to the carbonyl group, in this case there's only one such carbon ) source, leading to enolization, double bond migration, and loss of hydroxyl group to produce an alpha-beta unsaturated aldehyde.
This?
Precisely! :)
Post by: sjb on January 20, 2013, 04:25:01 PM
Post by: souro10 on January 21, 2013, 02:16:00 AM
Post by: neobenzene on January 26, 2013, 01:51:07 AM
Post by: souro10 on January 27, 2013, 07:30:27 AM
Post by: neobenzene on February 02, 2013, 10:23:25 AM
Post by: orgopete on February 02, 2013, 09:45:48 PM
Why won't it dehydrate?
The aldol addition product lacks an acidic proton, so elimination is similar to a dehydration of 2-butanol with base. the most acidic hydrogen would be the OH, further hampering elimination.
It would be difficult to predict exactly what will be the result. The aldol addition product could be the result, but other reactions might also occur with the aldehyde.
Post by: neobenzene on February 03, 2013, 04:44:01 AM
Quote
The aldol addition product lacks an acidic proton, so elimination is similar to a dehydration of 2-butanol with base. the most acidic hydrogen would be the OH, further hampering elimination.
It would be difficult to predict exactly what will be the result. The aldol addition product could be the result, but other reactions might also occur with the aldehyde.
Thanks, orgopete!