Post by: Vrig on January 20, 2013, 02:55:20 PM
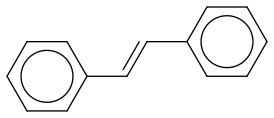
That's trans-stilben. The question is "Explain the sterochemical outcome in the bromination of trans-stilbene with Br2. Propose a synthesis of the opposite dibromide diastereomer". I understand why we get trans-dibromide relationship when letting bromine attack nucleophilicly. I don't know how to add the two bromine syn though. Also is it possible for stilben to rotate so that we have Ph-groups cis?
Or am I not understanding the question correctly?
Post by: curiouscat on January 20, 2013, 02:57:43 PM
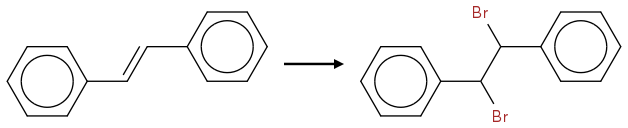
http://www.chemicalforums.com/index.php?topic=44068.0
Post by: Vrig on January 20, 2013, 03:15:23 PM
That's the bromination of trans-stilbene, right? My question is what does "the other diastereoisomer" look like? If I'm getting this at all the bromination shown above gives us (R,S) and I think they're asking for (R,R) or (S,S)? That would mean we have to add bromine syn to trans-stilben, right? How would we do this? With oxygen we could use osmiumtetraoxide, but is there such a thing for Br? And is it possible to rotate trans-stilben or will the phenyl groups (or Br) disallow rotation because of steric hindrance?
Sorry for my poor English...! :)
Post by: Vrig on January 20, 2013, 03:56:20 PM
You can lock the thread if you want to! :) Thanks