Post by: neobenzene on February 08, 2013, 11:59:25 PM
Now, what if the halide I'm using is benzyl chloride....benzene ring with a -CH2Cl attached to it. I'm not sure how to draw it using the SMILES engine.
Anyways, my question is, if we use benzyl chloride, and any sodium alkoxide (RONa), what will the product be? Will it be an ether, since the halide is primary, or will it be an alkene, since the halide has a bulky benzene ring?
Thanks!
Post by: discodermolide on February 09, 2013, 02:10:28 AM
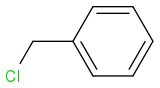
Post by: neobenzene on February 09, 2013, 03:22:45 AM
Post by: discodermolide on February 09, 2013, 03:36:23 AM
You cannot break the aromaticity.
You will not get
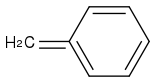
or
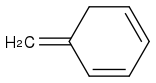
or
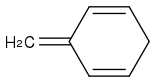
The first molecule does not make any sense.
Post by: neobenzene on February 09, 2013, 05:20:43 AM