Post by: Cooper on February 18, 2013, 08:17:29 PM
I was told that NBS always gave the most stable product. Is that true?
Even if it doesn't always, shouldn't you get the most stable (thermodynamic) product if you're adding heat, as in the reaction below?
AKA, why is my book telling me the lesser substituted alkene is the major product of this reaction?
Thanks
Post by: orgopete on February 18, 2013, 09:43:26 PM
Post by: Mocook on February 19, 2013, 12:05:42 AM
Post by: discodermolide on February 19, 2013, 01:34:09 AM
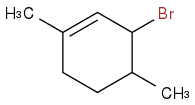
Post by: orgopete on February 19, 2013, 08:28:25 AM
It is correct , allylic bromination :)
In that case, could you give the journal reference for us.
Post by: Cooper on February 19, 2013, 01:55:38 PM
Post by: willug on February 19, 2013, 05:39:41 PM
Post by: Mocook on February 20, 2013, 08:07:05 PM
Post by: Cooper on February 20, 2013, 08:09:59 PM
is this not basic allylic bromination? this is pure theory due, bromine has a higher selectivity for tertiary carbons over secondary, therefore the major product is accurate.
NBS operates through radicals, which means high temperature. Therefore, the thermodynamic product should be the major product in normal cases. My professor said this is just because of weird substituent effects/compound shape and its an exception.
Post by: discodermolide on February 20, 2013, 08:31:08 PM
Post by: Cooper on February 21, 2013, 02:13:42 PM
Just to clarify, radicals do not automatically mean high temperature, you can generate them at low temperature as well.
My mistake. What conditions would you need to create radicals at low temperature? Light?
Post by: discodermolide on February 21, 2013, 02:26:02 PM
Also there are radical initiators which generate radical at quite low temperatures, by virtue of their instability.
Remember that biological radicals , mainly O2 are one of the initiators of allergic reactions, amongst other symptoms, ultimately causing death if not treated.
Post by: Cooper on February 21, 2013, 02:34:46 PM
Post by: discodermolide on February 21, 2013, 02:53:46 PM
The product I suggested is the most stable alkene and the most stable bromide.
Post by: Cooper on February 21, 2013, 02:54:31 PM