Post by: joyb on February 19, 2013, 08:46:27 AM
CH3CH=C(CN)2 + H2O → C2H4O + C3H2N2
The problem is on a sheet about addition to a C=C bond. I can't work out how you get two products with those molecular formulae. If anyone could help it would be much appreciated!
Post by: sjb on February 19, 2013, 08:54:23 AM
The question asks to identify the products and mechanism of the following reaction:
CH₃CH=C(CN)₂ + H₂O → C₂H₄O + C₃H₂N₂
The problem is on a sheet about addition to a C=C bond. I can't work out how you get two products with those molecular formulae. If anyone could help it would be much appreciated!
Can you format the numbers using [sub][/sub] tags (they're barely readable)? In any event, where are your nucleophiles / electrophiles? Is the first compound
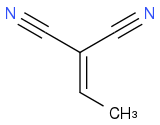 ?
?
Post by: joyb on February 19, 2013, 09:06:08 AM
Post by: orgopete on February 19, 2013, 09:48:55 AM
Post by: joyb on February 19, 2013, 10:02:03 AM
Post by: Dan on February 19, 2013, 10:19:05 AM
Have a look at your starting malononitrile. Is it a nucleophile/electrophile/acid/base?
What kind of reagent is water - nucleophile/electrophile/acid/base?
Post by: joyb on February 19, 2013, 03:26:30 PM
Post by: Dan on February 19, 2013, 03:53:10 PM
My original thought was that water would act as a nucleophile and that conjugate addition would occur due to the electron withdrawing nature of the CN groups.
Good. Half way there.
Hint: The pKa of H2(CN)2 is around 11
Post by: joyb on February 19, 2013, 06:57:51 PM
Post by: Dan on February 20, 2013, 02:55:53 AM
I don't really understand how you get from the reactants to the products.
I know that.
You are almost there, what you have suggested so far is correct, you just need to finish it.
You know you have this intermediate so far:
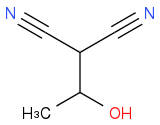
You know CH2(CN)2 (malononitrile) is one of the final products. Draw a circle around the part of the intermediate that corresponds to malononitrile. Which bond needs to break to make malononitrile?
Post by: joyb on February 20, 2013, 04:20:46 AM
Post by: Dan on February 20, 2013, 05:50:13 AM
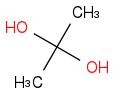
Post by: joyb on February 20, 2013, 06:03:02 AM
Post by: Dan on February 20, 2013, 06:11:04 AM
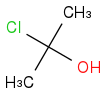
Post by: joyb on February 20, 2013, 07:06:00 AM
Post by: Dan on February 20, 2013, 07:51:00 AM
 :requil: acetone + HCl
:requil: acetone + HCl
Post by: orgopete on February 20, 2013, 12:03:06 PM
The question asks to identify the products and mechanism of the following reaction:
CH3CH=C(CN)2 + H2O → C2H4O + C3H2N2
The problem is on a sheet about addition to a C=C bond. I can't work out how you get two products with those molecular formulae. If anyone could help it would be much appreciated!
If C3H2N3 is malononitrile, then the starting material can be formed if the arrow for the above reaction is reversed.
Post by: camptzak on February 24, 2013, 12:50:37 AM
A lone pair on the alcohol group comes down and kicks off the carbon with the two cyanide groups and a hydrogen attached. The anion grabs another proton forming your C3H2N2.
Also you have your acetylaldehyde.
The acetylaldehyde and the hydrate would be in equilibrium with eachother.
wont the cyano groups move to carboxylic acid groups in water?