Post by: Rutherford on March 04, 2013, 10:52:48 AM
I need to finish this scheme:
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fimg19.imageshack.us%2Fimg19%2F7541%2F97580224.gif&hash=83c84d2fbff1459bbd267217160e639da75023aa)
knowing that A is benzene and B is propene. I got that C is isopropylbenzene, but I can't figure out what is D, if it produces bisphenol A with phenol. What is D and what is the reaction with phenol?
Post by: discodermolide on March 04, 2013, 11:24:46 AM
http://en.wikipedia.org/wiki/Bisphenol_A (http://en.wikipedia.org/wiki/Bisphenol_A)
Post by: Rutherford on March 04, 2013, 12:10:05 PM
Post by: discodermolide on March 04, 2013, 12:21:42 PM
http://en.wikipedia.org/wiki/Acetone (http://en.wikipedia.org/wiki/Acetone)
see if you can find any other links for example http://en.wikipedia.org/wiki/Cumene_process (http://en.wikipedia.org/wiki/Cumene_process)
Post by: Rutherford on March 05, 2013, 09:43:01 AM
Phenol reacts with nitric acid.
The molecule of G has two planes of symmetry (that of the molecule and an orthogonal one), while the plane of the molecule is the only element of symmetry for H. Starting with G, one can obtain paracetamol J via a two-stage process.
G should be the ortho isomer, while H should be the para isomer. Paracetamol has para -substituted groups, but G has ortho ones. Is this a mistake? The paracetamol should be obtained from H instead?
By reduction an amine is produced. Why will the amine group react with the anhydride, but not the hydroxy group?
Post by: discodermolide on March 05, 2013, 10:03:50 AM
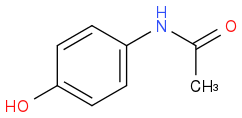
The amine is more nucleophillic that the OH therefore will react with the anhydride.
What exactly have you got for the structures of G & H?
Post by: DrCMS on March 05, 2013, 10:18:58 AM
The molecule of G has two planes of symmetry (that of the molecule and an orthogonal one), while the plane of the molecule is the only element of symmetry for H. Starting with G, one can obtain paracetamol J via a two-stage process.
G should be the ortho isomer, while H should be the para isomer. Paracetamol has para -substituted groups, but G has ortho ones. Is this a mistake? The paracetamol should be obtained from H instead?
Why do you think the otho isomer has 2 planes of symetry but the para only has one? I think you'll find the exact opposite is true.
Post by: Rutherford on March 05, 2013, 10:34:05 AM
Paracetamol isWhy? Why won't both react?
The amine is more nucleophillic that the OH therefore will react with the anhydride.
What exactly have you got for the structures of G & H?
Post by: discodermolide on March 05, 2013, 10:46:47 AM
Post by: Rutherford on March 05, 2013, 12:02:20 PM
L is a precursor of a dye Aluminon used for quantitative determination of aluminum and some other metals. Reaction of two equivalents of L with formaldehyde under acidic conditions affords N. Addition of one more equivalent of L to N in the presence of NaNO2 and sulfuric acid yields O , which finally gives Aluminon upon treatment with ammonia.
What is N and the reaction producing it?
I got that L is salicylic acid.
Post by: discodermolide on March 05, 2013, 12:11:38 PM
Post by: Rutherford on March 05, 2013, 01:03:54 PM
Two equivalents of an acid are required for acidification of
K to form compound L.
Post by: discodermolide on March 05, 2013, 01:17:39 PM
![OC1=CC=C(C([H])=O)C=C1C(O)=O](https://www.chemicalforums.com/SMILES/b3dc78d2c2f26bd11d15.png)
Post by: Rutherford on March 05, 2013, 01:27:42 PM
Okay.Two equivalents of L reacted.
L is a precursor of a dye Aluminon used for quantitative determination of aluminum and some other metals. Reaction of two equivalents of L with formaldehyde under acidic conditions affords N. Addition of one more equivalent of L to N in the presence of NaNO2 and sulfuric acid yields O , which finally gives Aluminon upon treatment with ammonia.
What is N and the reaction producing it?
I got that L is salicylic acid.
Post by: discodermolide on March 05, 2013, 01:33:57 PM
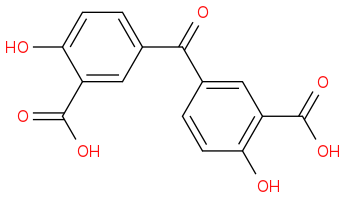
what is your suggestion for L?
Post by: Rutherford on March 05, 2013, 01:46:31 PM
I wrote that I got that L is salicylic acid.
Post by: discodermolide on March 05, 2013, 01:48:39 PM
How would that reaction happen?
I wrote that I got that L is salicylic acid.
Sorry I meant N
Post by: Rutherford on March 05, 2013, 01:52:40 PM
Post by: discodermolide on March 05, 2013, 02:00:06 PM
Have a look here.
http://en.wikipedia.org/wiki/Aurintricarboxylic_acid (http://en.wikipedia.org/wiki/Aurintricarboxylic_acid)
The reaction of 2 x L with formaldehyde and sulphuric acid to give N is surely a Friedel-Crafts acylation?
Post by: Rutherford on March 05, 2013, 02:41:19 PM