Post by: hblondie26 on May 07, 2013, 08:42:02 AM
(a) Which aromatic compound and organometallic reagent would you use in each of the preparations described above?
I have whittled it down to the following 2 possibilities -
C6H5CN + C5H10MgBr
C6H5COOH + C5H10Li
Please can you advise if I am on the right track or do I need to split the carbon and hydrogens to show the structure ie:
(CH33)22CCHCH22MgBr
Post by: Dan on May 07, 2013, 10:36:30 AM
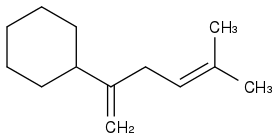
I assume what you meant was this?
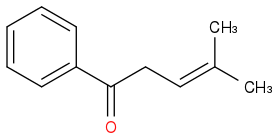
Post by: hblondie26 on May 07, 2013, 11:42:04 AM
On that basis are my workings out correct?
Post by: opsomath on May 07, 2013, 11:56:44 AM
Post by: hblondie26 on May 07, 2013, 12:18:31 PM
(CH3)2CCHCH2Li and C6H5COOH
Post by: discodermolide on May 07, 2013, 12:33:07 PM
Perhaps it may be worth considering an acid derivative?
Post by: hblondie26 on May 07, 2013, 01:01:24 PM
So (CH3)2CCHCH2LiAlH4 us the aromatic carboxylic acid?
Post by: discodermolide on May 07, 2013, 01:12:24 PM
Not an aluminium hydride.
Post by: hblondie26 on May 07, 2013, 01:46:20 PM
Post by: discodermolide on May 07, 2013, 01:53:03 PM
Post by: opsomath on May 07, 2013, 02:43:16 PM
Post by: hblondie26 on May 07, 2013, 07:50:51 PM
Post by: orgopete on May 08, 2013, 02:01:05 PM
Post by: opsomath on May 08, 2013, 02:06:25 PM
Post by: hblondie26 on May 08, 2013, 03:19:18 PM
I don't know what acid derivative to use unless you do something like
C6H5CH3COOH
WIth the nitrile I have used MgBr
Post by: opsomath on May 08, 2013, 03:33:49 PM
http://www.orgsyn.org/orgsyn/orgsyn/prepcontent.asp?prep=cv5p0775
Alternatively, you could make a Weinreb amide using an amine coupling procedure, then react it with an organolithium or similar.
http://en.wikipedia.org/wiki/Weinreb_ketone_synthesis#Preparation
Post by: orgopete on May 08, 2013, 09:50:42 PM
Well, I stand by my original answer; you can directly react an organometallic (including organolithiums) with a carboxylate, making a ketone after workup.
http://www.orgsyn.org/orgsyn/orgsyn/prepcontent.asp?prep=cv5p0775
Alternatively, you could make a Weinreb amide using an amine coupling procedure, then react it with an organolithium or similar.
http://en.wikipedia.org/wiki/Weinreb_ketone_synthesis#Preparation
I agree, those are possibilities. Would you think those reactions were part of the class the poster was taking?
Post by: opsomath on May 08, 2013, 09:59:25 PM
Post by: hblondie26 on May 12, 2013, 04:00:52 AM
I have copied and pasted the exact question and compound below.
Thank you for all the previous help.
Compound 5 can be prepared either by reacting an organometallic reagent with an aromatic nitrile, or by reacting a different organometallic reagent with an aromatic carboxylic acid (assume appropriate work up).
(a) Which aromatic compound and organometallic reagent would you use in each of the preparations described above?