Post by: Rutherford on August 22, 2013, 01:25:42 PM
For example:
If we treat the above compound in basic condition with Grignard the Grignard reagend would attack the less sterically hindered C atom which is the one right to the oxygen in the ring.
Then, if we treat the same compound with Grignard in acidic conditions, the oxygen gets protonated and the Grignard will attack the sterically hindered C atom because the transition state is more stable.
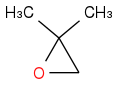
Now to the thing that confuses me. If we treat a compound like:
![CC1(C)[Br+]C1](https://www.chemicalforums.com/SMILES/8644f8bd7db9ab3e1884.png)
With OH- regardless of acid/base conditions it should attack the sterically hindered C atom because it has a bigger partial positive charge than the other C atom. This seems to be in contradiction with the previous examples. Why wouldn't Grignard attack that C atom because of the positive charge in the previous example? I need some clarification here.
Post by: discodermolide on August 22, 2013, 01:29:12 PM
You need to work out the mechanism of the three reactions you described, therein lies your answer.
See if you can come up with something.
Post by: Rutherford on August 22, 2013, 02:00:34 PM
Post by: AlphaScent on August 22, 2013, 02:15:28 PM
Water is not as bulky as the grignard and would add to the bromonium ion ans the hindered position to allow the bromine atom to be in less hindered environment for further displacement. This is just me rambling a bit and trying to make sense of it.
My one question to you Raderford is when would you do a grignard under acidic conditions?? It is a base and would react with said acid and do nothing, right?
Post by: discodermolide on August 22, 2013, 02:17:46 PM
Post by: Rutherford on August 22, 2013, 02:45:46 PM
The reagent. The first reaction is ok, the second, I would suggest that the ring opens first and the carbocations is attacked by waterIt's not that way presented in Vollhard but it would rationalize things.
This is a good question. I am trying to think of a orbital justification for this perceived mechanism.
Water is not as bulky as the grignard and would add to the bromonium ion ans the hindered position to allow the bromine atom to be in less hindered environment for further displacement. This is just me rambling a bit and trying to make sense of it.
My one question to you Raderford is when would you do a grignard under acidic conditions?? It is a base and would react with said acid and do nothing, right?
:o That reaction I made up, didn't want to change reagent for simplicity. Sorry for the silly mistake.
I like the idea of bromine being in a less hindered environment. But when epoxyde gets protonated it reacts in the same way as bromonium ion, so it can't be that.
Post by: AlphaScent on August 22, 2013, 04:16:05 PM
Post by: AlphaScent on August 22, 2013, 04:17:02 PM
Post by: Rutherford on August 22, 2013, 05:12:13 PM
Thanks to you two for solving this.
Post by: TwistedConf on August 22, 2013, 10:42:03 PM
Okay, I will remember it that way. It's best to take it as sN1.
It's actually not.
The exact nature of a bromonium ion is quite complex, and depends upon the structure of the ion itself (ie. what's attached to the two carbons) and other reaction variables.
In a case like the one you drew, experimental evidence suggests that a carbocation is not involved. For one thing, these ring opening reactions are stereoselective- so there's no rotation around the C-C bond in the intermediate. In related (but not identical) bromonium ions, favorable rearrangements are possible but not observed.
You're better off thinking of that intermediate as having a partial polarization at the more substituted carbon, which is then attacked from the back side, rather than an actual carbocation/SN1.
Post by: Rutherford on August 23, 2013, 05:06:24 AM
Post by: AlphaScent on August 23, 2013, 12:16:59 PM
Post by: Rutherford on August 23, 2013, 01:20:00 PM
As Twisted said, carbocations are not involved in bromonium reactions.
Post by: TwistedConf on August 23, 2013, 06:19:03 PM
Is a bromonium that much more complex than a protonated epoxide ion?
In the practical final product sense, not really. But comments above mentioned bromonium ring opening prior to attack and carbocations being involved in the process, and there's literature and experimental evidence out there that indicates otherwise- at least in cases like the one drawn in the original post.
Post by: TwistedConf on August 23, 2013, 07:55:24 PM
Why the epoxyde won't be attacked the same way?
They are attacked the same way, except when you have a really powerful nucleophile (like your Grignard) which doesn't require activation (protonation) of the oxygen and just attacks directly via the path of least resistance.
Post by: magician4 on August 23, 2013, 08:46:35 PM
I was reading a textbook and got confused with the reaction of oxacyclopropanes.
For example:
If we treat the above compound in basic condition with Grignard the Grignard reagend would attack the less sterically hindered C atom which is the one right to the oxygen in the ring.
Then, if we treat the same compound with Grignard in acidic conditions, the oxygen gets protonated and the Grignard will attack the sterically hindered C atom because the transition state is more stable.
what do you mean by "basic conditions" / "acid conditions" ?
in the presence of Grignard reagents, it is very difficult to have substances more basic than the Grignard - substance in its own right around ( BuLi , maybe) ...
... and those wouldn't interfer with the Grignard ./. epoxide situation at all, IMHO (except of competing, maybe)
if, on the other hand, you had an acid condition with an oxonium - ion (which would be most difficult to achieve , to start with), all that would happen was the destruction of the Grignard reagent:
R-MgX + [epoxonium]Y :rarrow: R-H + MgXY
to me, the example given hence seems to be completely meaningless
Now to the thing that confuses me. If we treat a compound like:same problem again: it is impossible to have something like a bromonium-structure under alkaline conditions
With OH- regardless of acid/base conditions it should attack the sterically hindered C atom because it has a bigger partial positive charge than the other C atom. This seems to be in contradiction with the previous examples. Why wouldn't Grignard attack that C atom because of the positive charge in the previous example? I need some clarification here.
... and under acid conditions, general expectation would be that the attacking particle would be water (and not hydroxy) and would be "added" (with subsequent release of H+) to the more stable carbocation (which is the tertiary , and not the secondary)
regards
Ingo
Post by: Rutherford on August 24, 2013, 05:47:28 AM
If they are attacked the same way, why in one case the less hindered C atom is attacked and in the other the more hindered C atom is attacked? That's again the original question I posted.Why the epoxyde won't be attacked the same way?
They are attacked the same way, except when you have a really powerful nucleophile (like your Grignard) which doesn't require activation (protonation) of the oxygen and just attacks directly via the path of least resistance.
Quote
what do you mean by "basic conditions" / "acid conditions" ?I just wanted to emphasize that in the first case the epoxyde won't be protonated and in the second that it will, and I wrote in one of my previous posts that I made a mistake when putting together acid and Grignard:
Me:
Quote
That reaction I made up, didn't want to change reagent for simplicity. Sorry for the silly mistake.
I like the idea of bromine being in a less hindered environment. But when epoxyde gets protonated it reacts in the same way as bromonium ion, so it can't be that.
No carbocation would be formed in the reaction of bromonium with H2O.
Post by: magician4 on August 24, 2013, 07:01:43 PM
Quote
I just wanted to emphasize that in the first case the epoxyde won't be protonated and in the second that it will, and I wrote in one of my previous posts that I made a mistake when putting together acid and Grignard (...)I just picked up your example, as it really doesn't matter what kind of negatively charged nucleophile will try to attack: they're all subject to the same fate
replace "Grigneard " with "cyanide" , for example , and the outcome would be the same
Quote
No carbocation would be formed in the reaction of bromonium with H2O.I think you're definitively wrong here insofar, as for the relevant moment of the reaction the "easiness" of the formation of a stabilized cation is of highest importance, and determines the orientation resulting thereof.
that's where all the difference you're wondering about originates from: that this pathway is relevant in case of -onium situation under acid conditions
...and irrelevant in all other cases, including the alkaline situation, where steric factors then become the name of the game.
you might not like my answer, you even might keep on giving me negative mole snacks for it again and again, but that's just what the chemical situation is like
regards
Ingo
Post by: Rutherford on August 25, 2013, 03:32:21 AM
Post by: Rutherford on August 25, 2013, 09:00:34 AM
Post by: magician4 on August 25, 2013, 02:36:21 PM
Quote
Why is it relevant in one situation and irrelevant in the other?[ let's stay with expoxide / oxonium for this (as I don't like the idea of a bromonium-ion under alkaline conditions):]
because we're talking different mechanisms in different situations leading to different products !
(i) attack of a negatively charged particle on the non-protonated epoxide (i.e. alkaline conditions)
here, the epoxide is quite stable and won't open up all by itself in any meaningfull degree (i.e. wouldn't form any carbocations, neither secondary nor tertiary, and hence will not react via SN1 )
hence, we need SN2 to achieve something, with the sterically less hindered side being the one most likely to be the center of the successfull action
(ii) addition of a neutral "nucleophile" on the protonated epoxide (with subsequent extrusion of H+)
oxonium (! the oxygen must be charged for this) being an excellent leaving group, there'll be an equilibrium of three (relevant) species present:
- the epoxide with the H+ bearing, hence positively charged, oxygen
- one open structure where the positive charge is located at the -CH2- group
- one open structure where the positive charge is located at the (CH3)2-C- group
from those three, only the last two are able to result in something meaningfull when adding the nucleophile, and the last one of those will be much much more likely by far (due to the methylgroups stabilizing this structure)
this statistical advantage will lead to the product described, when adding the nucleophile
there might be some minor neighbouring group effects still involved , though, as the hydroxygroup first will need to move away and make place for the nucleopile to rush in (from one side, not from the other), which could result in some kind of pseudo - stereoselectivity (if we studied respective species, like one with one of the two methyls being CD3- group in exchange)
Quote
I see that this is so complex that no accepted explanation exist.this is your opinion
I am deeply convinced to the opposite (as shown above), and am very confident that there must be a bunch of deep and deeper mechanistic studies out there somewhere, supporting my general analysis, with such a simple system (and hence inviting to those studies at that)
regards
Ingo
Post by: Rutherford on August 25, 2013, 03:18:23 PM
(Now talking only about reactions happening in acidic conditions.) What would happen if we had an optically active epoxyde? Would the configuration inverse or remain unchanged when the reaction happens?
Post by: magician4 on August 25, 2013, 03:56:34 PM
- as I said, there might be some "blocking" effects due to the hydroxylgroup that initially has to move away beforehand (at least for some directions from which the nucleophile might wish to reach the carbon) for the subsequent tertiary carbocation to react.
this is a statistical disadvantage to the respective "side"
- second, any very fast reaction at the tertiary cation taking place before the attached groups were able to revolve several (undefined) times around the XYC+-R' bond
hence , you might find some "incomplete" retention of stereochemistry here (I called it "pseudo" in my last post, as this is NOT due to some Walden inversion type effects)
regards
Ingo
Post by: Rutherford on August 26, 2013, 05:42:03 AM