Post by: webassignbuddy on September 18, 2013, 09:18:52 AM
For the bottom reaction, wouldn't the product be this (when the C=C double bond on the far right breaks as 2 electrons from it get pushed onto the top carbon, which then attacks the H-H and the H nucleophile attacks the electrophilic carbon):
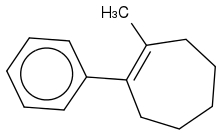
For the IUPAC I wrote, cis- 1-methyl-2-phenylcyclohept-1-ene (ene for the double bond at 1!) But that's wrong :(
The answer is supposed to be cis-1-methyl-2-phenylcycloheptane
I don't understand :(
1) Do BOTH C=C double bonds in cycloheptane get reduced to CH-CH because it's EXCESS H2/Pd? Also did I describe the Excess H2/Pd mechanism correctly?
2) Does the correct answer look like this?
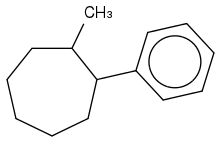
3) Why wouldn't I reduce all the double bonds in the benzene? Is it because H2/Pd catalyst in general can't because it's not a strong enough reagent and it's only used for alkenes?
4) How exactly do you name a structure like this? I know that cis means 2 things are on the SAME side of a bond (hence on the far right of the cycloheptane structure, the H's are sticking OUT on the same side of the C-C bond because there's a lot of space/elbow room, and on the top left of the cyclheptane structure, the H's would be sticking INTO the page while being on the same side of the C-C bond because it's crowded). Is my reasoning correct?
Post by: discodermolide on September 18, 2013, 09:28:38 AM
Reduction of the aromatic ring requires either special catalysts or pressure and temperature.
Post by: webassignbuddy on September 18, 2013, 09:36:38 AM
I variability hydrogenation of such double bonds gives the cis product. Imagine the molecule approaching the catalyst surface. It can only do so when both substituents point up out of the plane. This gives the cis product.
Reduction of the aromatic ring requires either special catalysts or pressure and temperature.
What do you mean by "I variability hydrogenation of such double bonds" though?
Also, why would the top reaction in the picture below not be called cis-1,3-diphenylheptane (https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fwww.webassign.net%2Fuserimages%2Fkasandbe%40ncsu%2FCH223_fall_02%2F223fall13_IW2_3.gif&hash=26e1263b941294b5a26523a18d1965d7d38da4e6)
The correct answer is supposed to be just 1,3-diphenylheptane.
I don't understand :( You only put CIS or TRANS if there is a CYCLIC structure containing C=C double bonds??
Post by: discodermolide on September 18, 2013, 09:47:46 AM
It should be invariably.
Post by: webassignbuddy on September 18, 2013, 09:58:17 AM
Sorry the spell checker changed it.
It should be invariably.
Ok so if there was just 1 C=C double bond in cyclopentane, would it still be cis?
Also do you only put cis or trans if there is a cyclic structure containing C=C double bonds??
Post by: discodermolide on September 18, 2013, 10:03:30 AM
You also use cis and trans in non cyclic structures.
In the case of the top reaction you probably get mixtures after the hydrogenation, mostly trans and some cis. So then you don't define it as cis or trans.
Post by: webassignbuddy on September 18, 2013, 10:05:46 AM
In cyclopentene the double bond is cis by default.
You also use cis and trans in non cyclic structures.
In the case of the top reaction you probably get mixtures after the hydrogenation, mostly trans and some cis. So then you don't define it as cis or trans.
Hm...ok, I think I understand
Thanks
Post by: webassignbuddy on September 18, 2013, 10:16:19 AM
In cyclopentene the double bond is cis by default.
You also use cis and trans in non cyclic structures.
In the case of the top reaction you probably get mixtures after the hydrogenation, mostly trans and some cis. So then you don't define it as cis or trans.
For the second reaction, the answer is
4-chloro-4-methyl-1,7-diphenylheptane. But My question is, for the HCl reaction at the bottom of the diagram I posted above your most recent post is why does the Chlorine attack the most substituted carbon at 4? Why not carbon 5 on top?
Post by: discodermolide on September 18, 2013, 10:24:29 AM
Post by: webassignbuddy on September 18, 2013, 11:18:46 AM
protonation of the double bond will produce the most stable carbonic ion, that then is substituted by Cl.
oh ok...so the most substituted carbon
Post by: discodermolide on September 18, 2013, 11:29:03 AM
Post by: antimatter101 on September 21, 2013, 02:07:21 AM
Also, H3C- is spelt as 'carbanion'.
Post by: hinhthoi on September 24, 2013, 02:15:52 AM
Post by: discodermolide on September 24, 2013, 04:27:28 AM
carbanion and carbonium ion are the same thing
Just to clarify a carbanion is a carbon bearing a negative charge, usually stabilized by resonance, but it does not have to be.
A carbonium ion is a carbon bearing a positive charge resonance stabilised.
Post by: IA_khim on September 25, 2013, 05:21:52 PM