Post by: Altered State on October 16, 2013, 09:30:08 AM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2F1wCpfE2.png&hash=adb66eeb271bf411e473e1eba3683174ded1a972)
M would be the acyl chloride I've drawn. Then we add 2 equivalents of diazomethane. The first equivalent will give the compound M-N (addition to carbonyl, chlorine leaves, and then attacks the new carbon liberating nitrogen gas).
Now is when the trouble starts. I see quite a few ways the reaction could follow. The first one is that diazomethane just attacks carbon attached to chlorine, and we add another carbon to the molecule, obtaining compound N3.
The other way is that diazomethane's carbon attacks carbonyl, giving the intermediate MNN. That intermediate would be very unstable, and I think it would go to compound N1. But in both N1 and N3 we have a problem, the next step. Next step is adding an alkene under hv conditions. I believe that this last reaction is supposed to be the formation of a carbene, and then addition to alkene to give a cyclopropane.
Ok, that sounds logic, but the only way that could happen is that we have as compound N a molecule tat still has a -N2 susbtituent. That gives me 2 possibilities, one is N2 (don't think so, so unstable compound) and a compound like N3 but with a -N2 instead of chlorine.
The problem is that I don't see how that could happen with free chlorines all around capable of pushing N2 out of the molecule, or in the epoxide case, the oxygen.
Where does my reasoning fail?
Post by: discodermolide on October 16, 2013, 09:34:38 AM
Perhaps think about a Wolff rearrangement?
Post by: Altered State on October 16, 2013, 09:51:56 AM
After the addition of diazomethane to the acid chloride you have a alpha-diazo ketone.
Perhaps think about a Wolff rearrangement?
I didn't know that rearrangement, I've checked it and that would be the product:
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi.imgur.com%2FW1FK01o.png&hash=bacbcfa57536745b8915525c4e73ae71b1bc1eb8)
That with another equivalent, I suppose it would lead to that epoxide, that won't react with an alkene under hv conditions. I still have the same problem..
Post by: discodermolide on October 16, 2013, 10:05:53 AM
Post by: Altered State on October 16, 2013, 10:41:02 AM
Carbenes, formed from diazo compounds, also add to double bonds to give cyclopropanes.
I know, the problem is that I don't come up with a compound N that is a diazo compound
Post by: Altered State on October 17, 2013, 09:34:35 AM
I don't usually ask for homework, but I need this one due tomorrow this time.
Post by: discodermolide on October 17, 2013, 10:12:25 AM
to give
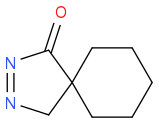
Post by: Altered State on October 17, 2013, 10:46:49 AM
Would the second equivalent do a 1,3-cycloaddition with the ketene?
to give
That could make sense, but I've definetly never been taught cycloadditions (except diels-alder), and furthermore, I if it were the case, would be possible to react that compound with an alkene to form cyclopropane?
Post by: discodermolide on October 17, 2013, 11:05:31 AM
Post by: Altered State on October 17, 2013, 01:59:19 PM
Post by: Dan on October 17, 2013, 03:52:51 PM
Post by: orgopete on October 17, 2013, 05:13:59 PM
The problem may not have been well formulated. If the book or class discussed the addition, then okay otherwise I think you need to work through this very carefully. If diazomethane were added to RCOCl, what are the products? Hint, a neutral plus a neutral gives? Hint #2, I think the second diazomethane results in methyl chloride.