Post by: webassignbuddy on November 26, 2013, 11:58:23 AM
Draw only the deprotonated product and that WITHOUT any spectator ion.
I put in
 as my answer but got it wrong.
as my answer but got it wrong.:(
Help?
Post by: discodermolide on November 26, 2013, 12:02:04 PM
So which compound will be the most acidic?
Post by: webassignbuddy on November 26, 2013, 12:17:15 PM
In your answer you forgot the OH group!
So which compound will be the most acidic?
I was thinking the third one will be the most acidic. And OH leaves according the the diagram.
Post by: webassignbuddy on November 26, 2013, 12:26:22 PM
Post by: webassignbuddy on November 26, 2013, 12:33:34 PM
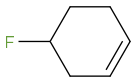
Post by: discodermolide on November 26, 2013, 12:35:44 PM
And I do not agree that the third compound will be the most acidic.
Compare cyclohexanol to phenol in their reactions with OH-, which will be de-protonated faster?
Post by: discodermolide on November 26, 2013, 12:36:30 PM
Is the answer this?
No, that is not correct, see my previous post.
Post by: webassignbuddy on November 26, 2013, 12:39:15 PM
Is the answer this?
No, that is not correct, see my previous post.
![[O-]c1ccc(F)cc1](https://www.chemicalforums.com/SMILES/fd37cf8108f2a088aae8.png)
Post by: AlphaScent on November 26, 2013, 12:39:48 PM
Have you come to a conclusion? Reasoned anything out as to why it is not the third compound?
Post by: AlphaScent on November 26, 2013, 12:40:33 PM
Why might it be that is the most easily deprotonated?
Post by: webassignbuddy on November 26, 2013, 12:41:50 PM
Is that a guess?
Why might it be that is the most easily deprotonated?
Because F is withdrawing electron density from the stable/electron rich benzene ring. Which causes electron denesity of the OH to be donated INTO the benzene ring to compensate. Thus making the O-H bond really thin and the H really acidic/very likely to be deprotonated.
Post by: discodermolide on November 26, 2013, 12:48:20 PM
Post by: AlphaScent on November 26, 2013, 04:05:31 PM