Post by: joshphillips1977 on December 08, 2013, 07:04:37 PM
C
|
C-C-C-C-C
|
C
I came up with 3-propylpentane, would that be correct?
Post by: discodermolide on December 08, 2013, 07:09:28 PM
Post by: joshphillips1977 on December 08, 2013, 07:11:26 PM
Post by: discodermolide on December 08, 2013, 07:15:13 PM
Post by: joshphillips1977 on December 08, 2013, 07:19:44 PM
|
C
|
C-C-C-C-C-C
| |
C C
|
C
|
C
I came up with 2 propyl 2-butyl 4-ethylhexane, but am unsure if this is correct?
Post by: joshphillips1977 on December 08, 2013, 07:23:38 PM
3-prop
Post by: discodermolide on December 08, 2013, 07:26:18 PM
Post by: joshphillips1977 on December 08, 2013, 07:32:05 PM
Post by: discodermolide on December 08, 2013, 07:35:15 PM
Post by: joshphillips1977 on December 08, 2013, 07:41:08 PM
Post by: discodermolide on December 08, 2013, 07:42:55 PM
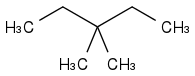
is 3,3-dimethylpentane
Post by: joshphillips1977 on December 08, 2013, 07:47:58 PM
Post by: discodermolide on December 08, 2013, 07:49:14 PM
Post by: Arkcon on December 08, 2013, 07:50:56 PM
Post by: joshphillips1977 on December 08, 2013, 07:52:28 PM
Post by: discodermolide on December 08, 2013, 07:55:52 PM
Post by: joshphillips1977 on December 08, 2013, 08:06:43 PM
Post by: discodermolide on December 08, 2013, 08:09:31 PM
3,3-dimethylpentane, di meaning two!
Post by: joshphillips1977 on December 08, 2013, 08:18:40 PM
Post by: discodermolide on December 08, 2013, 08:22:35 PM
C-C-C-C-C on the middle carbon there are two substituents two methyl groups.
the longest chain is 5 C atoms i.e. a pentane, on the middle carbon number 3 are two methyl substituents, i,e, dimethyl.
so you have 3,3-dimethylpentane.
No magic, that's it.
Post by: joshphillips1977 on December 08, 2013, 08:25:54 PM
C
|
C
|
C-C-C-C-C-C
| |
C C
|
C
|
C
Which makes this one 2-propyl 2ethyl 4methylHexane Right? could I say 2,2,4 propylethylmethylHexane?
Post by: discodermolide on December 08, 2013, 08:32:05 PM
Post by: joshphillips1977 on December 08, 2013, 08:35:19 PM
Post by: discodermolide on December 08, 2013, 08:37:26 PM
Back to the other one, the two "3"s, this is because the two substituents are on the same carbon, carbon number 3, hence 3,3-dimethyl.
Post by: discodermolide on December 08, 2013, 08:44:39 PM
Post by: joshphillips1977 on December 08, 2013, 08:45:46 PM
Post by: discodermolide on December 08, 2013, 08:47:36 PM
Now what about the other one, look at the picture I posted.
Post by: joshphillips1977 on December 08, 2013, 08:47:48 PM
see picture.Oh Snap I get what your saying now... so i am gonna try to answer the second one again and repost...
Post by: joshphillips1977 on December 08, 2013, 08:50:31 PM
see picture.
4,6 dimethyl 4-ethylOctane??
Post by: discodermolide on December 08, 2013, 08:57:10 PM
the substituents go in alphabetical order.
Then the sum of the numbers must be the lowest, that is in your name the substituents are not in alphabetical order, and the numbers add up to 14.
In my name they are in alphabetical order and the sum is 13.
Post by: joshphillips1977 on December 08, 2013, 09:08:41 PM
Post by: discodermolide on December 08, 2013, 09:12:20 PM
Post by: joshphillips1977 on December 08, 2013, 09:24:16 PM