Post by: SeriouSyrius on January 05, 2014, 02:32:35 AM
I was using benzene to convert it to 4-hydroxybenaldehyde and then I used cyclohexane to convert it to Cyclohexanone.
1 mole of Cyclohexanone + 2 moles of 4-hydroxybenaldehyde ---NaOH(aq)---> (2E, 6E)-2,6-bis(4-hydroxybenzylidene) cyclohexanone
Is this right?
Post by: discodermolide on January 05, 2014, 04:31:06 AM
The latter compound is commercially available.
Post by: SeriouSyrius on January 05, 2014, 06:17:39 AM
Post by: discodermolide on January 05, 2014, 10:37:39 AM
You have an aromatic aldehyde in the presence of conc. NaOH at high temperature, what other reaction could occur here?
Post by: Altered State on January 05, 2014, 11:04:28 AM
Post by: SeriouSyrius on January 06, 2014, 06:18:21 AM
The last reaction to make the phenol, how do you see this proceeding?
You have an aromatic aldehyde in the presence of conc. NaOH at high temperature, what other reaction could occur here?
I got the last reaction from my notes. It says "Sodium Phenoxide + HCl(H+)---->Phenol"
As for the aromatic aldehyde with NaOH I think Aldol Reaction will occur? So I guess this part is wrong.
How about this method? I think i used diazonium salt to get OH.
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi44.tinypic.com%2F2nr2u8.jpg&hash=f2142105171cf2a873c07fc1b10ab8d5166f0ef6)
Post by: discodermolide on January 06, 2014, 07:19:41 AM
How do you think this proceeds?
Aromatic aldehydes undergo a named reaction in the presence of strong base, can you think of it? Named after a famous Italian chemist from the 1800s.
Post by: SeriouSyrius on January 06, 2014, 07:46:33 AM
I meant the substitution of Cl with OH.
How do you think this proceeds?
Aromatic aldehydes undergo a named reaction in the presence of strong base, can you think of it? Named after a famous Italian chemist from the 1800s.
I don't really get what you mean. All i know is the substitution of Cl with OH is to get alcohol and so I can reduce the primary alcohol to aldehyde. For the reaction name, I got no idea. All I can think of is Aldol Reaction.
Post by: discodermolide on January 06, 2014, 07:52:50 AM
I was just wondering if you knew how the substitution of Cl for OH worked (the reaction mechanism).
Post by: SeriouSyrius on January 06, 2014, 07:56:57 AM
The reaction I am talking about is the Cannizzaro reaction of aromatic aldehydes to give the benzyl alcohol and the benzoic acid. http://en.wikipedia.org/wiki/Cannizzaro_reaction (http://en.wikipedia.org/wiki/Cannizzaro_reaction)
I was just wondering if you knew how the substitution of Cl for OH worked (the reaction mechanism).
Oh. I didn't hear before Cannzizzaro reaction. I'm still studying for Organic Chemistry. Currently studying about Benzene, Phenol, Aniline and Aldol Condensation. So how was my method of getting (2E, 6E)-2,6-bis(4-hydroxybenzylidene) cyclohexanone? It's a really important assignment and I need to try to get much information about it as I can. This is my overall method:
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi43.tinypic.com%2F2qu2910.jpg&hash=3a0622b67699cbdb7e71c01f9eb32b9c4a8c9b3d)
Post by: discodermolide on January 06, 2014, 08:06:36 AM
The mechanism I was talking about, Cl going to OH (the original you showed) may go as I suggest in the attachment.
However I believe I am a bit ahead of your classes, sorry for any confusion I have caused. Anyway you have learnt a new reaction for yourself, and that's good.
Post by: SeriouSyrius on January 06, 2014, 08:14:26 AM
The new method certainly avoids problems with the Cannizzaro reaction. Using the diazonium salt is better.
The mechanism I was talking about, Cl going to OH (the original you showed) may go as I suggest in the attachment.
However I believe I am a bit ahead of your classes, sorry for any confusion I have caused. Anyway you have learnt a new reaction for yourself, and that's good.
It's alright. So if I'm not wrong the first overall method is alright but it's just that I have to use the method you attached? And the second method is also right?
Post by: discodermolide on January 06, 2014, 08:17:26 AM
Post by: SeriouSyrius on January 06, 2014, 08:19:40 AM
Post by: discodermolide on January 06, 2014, 08:24:06 AM
Post by: SeriouSyrius on January 06, 2014, 08:25:41 AM
That is the aldol reaction. This is a standard method for the preparation of such compounds. Analogues of curcumin, an interesting compound for various pharmacological applications, cancer for example.
Alright. Thanks! Helped a lot. :) :) :)
Post by: kriggy on January 07, 2014, 11:40:36 AM
Post by: SeriouSyrius on January 11, 2014, 08:28:27 AM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi42.tinypic.com%2Fm8mef9.jpg&hash=8d0a1100099a7e2c2d174fc6f496f6e1e81156e5)
As for the picture below, I was asked to think about the 3 side products but after reading the notes I had about aldol reaction, I can't seem to find the side products. All I can think of is the mixed aldol reaction but the problem now is there are 0 alpha-hydrogen in 4-hydroxybenzaldehyde and 4 alpha-hydrogen in cyclohexanone. How does the mixed aldol reaction work when 4-hydroxybenzaldehyde have 0 alpha-hydrogen???
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi39.tinypic.com%2Fofzihx.jpg&hash=51da77ecd79eb36f8dc391edc1ce5ca7bead09bd)
Post by: discodermolide on January 11, 2014, 08:52:17 AM
You take the product from the aldol of cyclohexanone and the benzaldehyde and react it with cyclohexanone. See if you can come up with the other possibilities.
I'm sorry but your new scheme is worse than the one you gave in. The acid chloride of formic acid, I'm not sure it exists. Try and find a route by making a benzoate from benzene.
Post by: SeriouSyrius on January 11, 2014, 09:52:11 AM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi43.tinypic.com%2F301euty.jpg&hash=ad9375a9cf8c4bb115fb0a921b493ddae690de23)
Post by: discodermolide on January 11, 2014, 10:14:47 AM
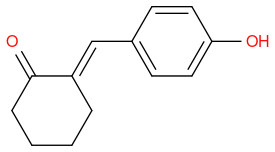
and can undergo an aldol with the aldehyde or cyclohexanone.
Post by: SeriouSyrius on January 11, 2014, 11:03:08 AM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi39.tinypic.com%2F25r2lb9.jpg&hash=7333753e9b0c151b2bb8447fdbbde0b352c4e7fd)
What about the previous picture I send? The side product I written there.
Post by: discodermolide on January 11, 2014, 11:12:23 AM
Post by: SeriouSyrius on January 11, 2014, 11:23:00 AM
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi44.tinypic.com%2F5frk2v.png&hash=9b3ea1928ecbda1506a917d2ac5bb5b27c94f3a7)
If that is not one of it, how about I switch the 2 side around but I don't think it make a difference? :/
(https://www.chemicalforums.com/proxy.php?request=http%3A%2F%2Fi44.tinypic.com%2F2uz54bp.jpg&hash=8340e344d456f924af6b4faeae3724e8b8bb996d)