Post by: Rutherford on February 15, 2014, 10:25:43 AM
Also: Explain why the C=O stretching frequency in 1,1’-carbonyldiimidazole is 100 cm–1 higher than that of 1,1’-carbonyldipyrrolidine.
I thought that the participation in conjugation would reduce the second bond character of the C=O bond, and move it lower, not higher. Where am I wrong at?
Post by: discodermolide on February 15, 2014, 10:30:10 AM
See if you can draw the structure and find out what happens next.
Post by: Rutherford on February 15, 2014, 10:34:09 AM
Post by: discodermolide on February 15, 2014, 10:47:02 AM
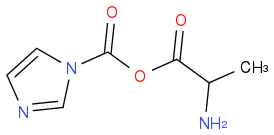
plus imidazole.
God knows what A is.
Post by: Rutherford on February 15, 2014, 10:52:48 AM
Post by: discodermolide on February 15, 2014, 10:59:46 AM
The other intermediates are ??
So F is probably the ionic intermediate?.
Never seen something like this before, strange scheme.
Post by: Rutherford on February 15, 2014, 11:07:37 AM
Post by: discodermolide on February 15, 2014, 11:13:40 AM
Post by: Rutherford on February 15, 2014, 11:20:41 AM
At the end activated Ala is produced, and the amino group of Gly can attack it and form Ala-Gly dipeptide right?
Post by: discodermolide on February 15, 2014, 11:23:20 AM
Logic, who applies logic? You can ask where do the protons come from. What starts the whole process off? Protonation of a carbonyl by an amino acid? You could go on for years with this and similar mechanisms.
Post by: Rutherford on February 15, 2014, 11:27:31 AM
Post by: discodermolide on February 15, 2014, 11:30:34 AM
Post by: Rutherford on February 15, 2014, 11:34:58 AM
Post by: discodermolide on February 15, 2014, 11:41:59 AM
Post by: Rutherford on February 15, 2014, 11:45:33 AM
Post by: discodermolide on February 15, 2014, 11:48:03 AM
Post by: Rutherford on February 15, 2014, 11:49:20 AM
Post by: discodermolide on February 15, 2014, 11:50:23 AM
Post by: Rutherford on February 15, 2014, 11:51:36 AM
Post by: orgopete on February 16, 2014, 09:15:55 AM
this is what I mean
While that could be the mechanism, I don't think it is. An imidazolium anion is fairly basic. I think the key is elimination of a neutral imidazole. The mechanism posted by Raderford was virtually correct, except the wrong nitrogen was protonated. If an amino acid protonated the imidazole, it would free the amino group and make neutral imidazole the leaving group. The other option is to write a concerted proton abstraction, but that will put an entropy load on the reaction.