Post by: Rutherford on April 23, 2014, 11:21:39 AM
Deduce the structure of an organic compound which has the following spectra:
Post by: Corribus on April 23, 2014, 11:56:37 AM
As no problem of the week anymore,Yeah, what is up with that, anyway?
Post by: Rutherford on April 23, 2014, 12:40:16 PM
Post by: discodermolide on April 23, 2014, 01:00:14 PM
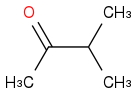
Post by: Rutherford on April 23, 2014, 01:31:19 PM
From the mass spectra, M=86g/mol and we can deduce the -CH3 and -C3H7, -C and -O fragments. This is in agreement with the C-NMR spectrum. The shift of the C atom above 200ppm means that one C atom is connected to oxygen. From H-NMR it can be seen that the -C3H7 group is isopropyl. Using the rest of the information from H-NMR one will end up with 3-methyl-2-butanone.
May you now post another problem you want in a new topic, so we others try to solve it?
Post by: Borek on April 23, 2014, 01:58:33 PM
Borek stopped with that because of low activity.
Yep. But I have nothing against some new interesting problems.