Post by: Partha Sarker on September 11, 2014, 02:51:32 AM
Then why it is called Carbolic Acid??
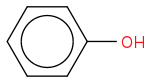
Post by: Borek on September 11, 2014, 02:59:13 AM
Post by: Partha Sarker on September 11, 2014, 03:03:35 AM
Acid is not a synonym of a "carboxylic acid". Acid means a substance that dissociates producing H+ and lowering pH of the solution.Oh! what happens when Phenol is mixed with water(chemical reaction please) and is there any other alcohol which does the same thing
Post by: Arkcon on September 11, 2014, 04:56:46 AM
Post by: Hunter2 on September 13, 2014, 10:09:57 AM
Post by: AdiDex on September 13, 2014, 01:01:24 PM
1. It can gives (Donate) H+ Ion ( A proton ).
2. It can accept an Electron.
And just remember that -
In general , A Neutral molecule consisting of Hydrogen which is connected with a electronegative atom is acidic in nature. ( As in case of Phenol , H is connected with O )