Post by: cseil on September 30, 2014, 12:56:44 PM
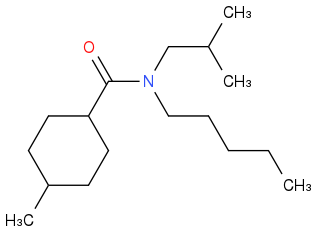
I'm starting with Organic Chemistry and the nomenclature. I just invented a structure, but the freeware version of ChemSketch doesn't tell me the name of molecules with more than 50 atoms, so I'm looking for a confirm.
It is a tertiary amine. It is bound to a pentane and to a 2-methylpropane.
So it is:
N-2-methylpropyl-N-pentyl-.
The first atom of the cycle is the one bound to the -COOH. So we have a 4-methylcyclohexane.
The name should be:
N-2-methylpropyl-N-pentyl-4-methylcyclohexanecarboxiamide.
Am I right?
I'm just interested into a hypothetic name.
PS. I'm italian, I don't really know if the order is different in some parts (for example butanoic anhydride is "anidride butanoica" in italian)
Post by: discodermolide on September 30, 2014, 01:11:00 PM
N-isobutyl-4-methyl-N-pentylcyclohexanecarboxamide
Isobutyl and 2-methylpropyl and equally correct. Some of the trivial names are allowed by IUPAC.
Post by: cseil on September 30, 2014, 01:20:23 PM
N-isobutyl-4-methyl-N-pentylcyclohexanecarboxamide
and not
N-isobutyl-N-pentyl-4-methylcyclohexanecarboxamide??
Post by: discodermolide on September 30, 2014, 01:45:10 PM
Post by: AromaticAcrobatic on September 30, 2014, 11:29:18 PM
wheres the tertiary amine?
Also,
-COOH is a specific functional group, as far as I can see you don't have this functional group within your molecule.
I hope I'm not coming off like a dick, but if you can recognize these things now you'll have a much better time in a couple weeks/months..
:D
Post by: cseil on October 01, 2014, 12:19:50 PM
I was wrong with the -COOH of course, but I didn't consider it as a -COOH so it was just distraction.
I have an amide when a -COOH group has a -OH replaced by an amine, right?
Isn't that tertiary?
Where was I wrong?
Thank you for your answer. I really appreciate it ;D
Post by: AromaticAcrobatic on October 01, 2014, 01:36:47 PM
You are right about the Amide but generally you don't consider that a tertiary amine, its an amide. The reason being is a tertiary amine is going to have different properties then an amide, so you want to keep them separate.
;)